BMP-regulated exosomes from Drosophila male reproductive glands reprogram female behavior
- PMID: 25154396
- PMCID: PMC4151142
- DOI: 10.1083/jcb.201401072
BMP-regulated exosomes from Drosophila male reproductive glands reprogram female behavior
Abstract
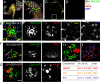
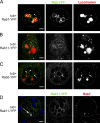
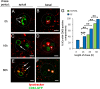
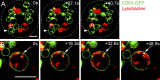
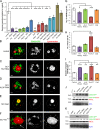
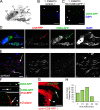
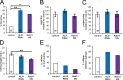
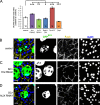
Male reproductive glands secrete signals into seminal fluid to facilitate reproductive success. In Drosophila melanogaster, these signals are generated by a variety of seminal peptides, many produced by the accessory glands (AGs). One epithelial cell type in the adult male AGs, the secondary cell (SC), grows selectively in response to bone morphogenetic protein (BMP) signaling. This signaling is involved in blocking the rapid remating of mated females, which contributes to the reproductive advantage of the first male to mate. In this paper, we show that SCs secrete exosomes, membrane-bound vesicles generated inside late endosomal multivesicular bodies (MVBs). After mating, exosomes fuse with sperm (as also seen in vitro for human prostate-derived exosomes and sperm) and interact with female reproductive tract epithelia. Exosome release was required to inhibit female remating behavior, suggesting that exosomes are downstream effectors of BMP signaling. Indeed, when BMP signaling was reduced in SCs, vesicles were still formed in MVBs but not secreted as exosomes. These results demonstrate a new function for the MVB-exosome pathway in the reproductive tract that appears to be conserved across evolution.
© 2014 Corrigan et al.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

