Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin
- PMID: 25154397
- PMCID: PMC4151150
- DOI: 10.1083/jcb.201401070
Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin
Abstract
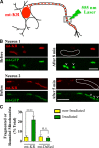
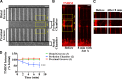
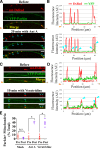
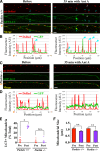
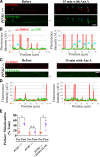
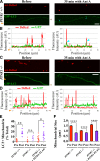
To minimize oxidative damage to the cell, malfunctioning mitochondria need to be removed by mitophagy. In neuronal axons, mitochondrial damage may occur in distal regions, far from the soma where most lysosomal degradation is thought to occur. In this paper, we report that PINK1 and Parkin, two Parkinson's disease-associated proteins, mediate local mitophagy of dysfunctional mitochondria in neuronal axons. To reduce cytotoxicity and mimic physiological levels of mitochondrial damage, we selectively damaged a subset of mitochondria in hippocampal axons. Parkin was rapidly recruited to damaged mitochondria in axons followed by formation of LC3-positive autophagosomes and LAMP1-positive lysosomes. In PINK1(-/-) axons, damaged mitochondria did not accumulate Parkin and failed to be engulfed in autophagosomes. Similarly, initiation of mitophagy was blocked in Parkin(-/-) axons. Our findings demonstrate that the PINK1-Parkin-mediated pathway is required for local mitophagy in distal axons in response to focal damage. Local mitophagy likely provides rapid neuroprotection against oxidative stress without a requirement for retrograde transport to the soma.
© 2014 Ashrafi et al.
Figures



Comment in
-
Parkinson's disease: A disorder of axonal mitophagy?Mov Disord. 2014 Nov;29(13):1582. doi: 10.1002/mds.26047. Epub 2014 Sep 25. Mov Disord. 2014. PMID: 25256171 No abstract available.
-
Neuronal mitophagy: long-distance delivery or eating locally?Curr Biol. 2014 Oct 20;24(20):R1006-8. doi: 10.1016/j.cub.2014.09.037. Epub 2014 Oct 21. Curr Biol. 2014. PMID: 25442848 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

