Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor
- PMID: 25154820
- PMCID: PMC4322257
- DOI: 10.1200/JCO.2014.56.2025
Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor
Abstract
Purpose: T cells can be genetically modified to express an anti-CD19 chimeric antigen receptor (CAR). We assessed the safety and efficacy of administering autologous anti-CD19 CAR T cells to patients with advanced CD19(+) B-cell malignancies.
Patients and methods: We treated 15 patients with advanced B-cell malignancies. Nine patients had diffuse large B-cell lymphoma (DLBCL), two had indolent lymphomas, and four had chronic lymphocytic leukemia. Patients received a conditioning chemotherapy regimen of cyclophosphamide and fludarabine followed by a single infusion of anti-CD19 CAR T cells.
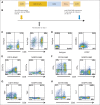
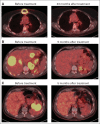
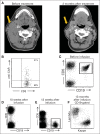
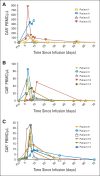
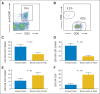
Results: Of 15 patients, eight achieved complete remissions (CRs), four achieved partial remissions, one had stable lymphoma, and two were not evaluable for response. CRs were obtained by four of seven evaluable patients with chemotherapy-refractory DLBCL; three of these four CRs are ongoing, with durations ranging from 9 to 22 months. Acute toxicities including fever, hypotension, delirium, and other neurologic toxicities occurred in some patients after infusion of anti-CD19 CAR T cells; these toxicities resolved within 3 weeks after cell infusion. One patient died suddenly as a result of an unknown cause 16 days after cell infusion. CAR T cells were detected in the blood of patients at peak levels, ranging from nine to 777 CAR-positive T cells/μL.
Conclusion: This is the first report to our knowledge of successful treatment of DLBCL with anti-CD19 CAR T cells. These results demonstrate the feasibility and effectiveness of treating chemotherapy-refractory B-cell malignancies with anti-CD19 CAR T cells. The numerous remissions obtained provide strong support for further development of this approach.
Trial registration: ClinicalTrials.gov NCT00924326.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Are all chimeric antigen receptors created equal?J Clin Oncol. 2015 Feb 20;33(6):651-3. doi: 10.1200/JCO.2014.57.5472. Epub 2015 Jan 20. J Clin Oncol. 2015. PMID: 25605860 No abstract available.
References
-
- Elstrom RL, Martin P, Ostrow K, et al. Response to second-line therapy defines the potential for cure in patients with recurrent diffuse large B-cell lymphoma: Implications for the development of novel therapeutic strategies. Clin Lymphoma Myeloma Leuk. 2010;10:192–196. - PubMed
-
- Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011:498–505. - PubMed
-
- Moore S, Kayani I, Peggs K, et al. Mini-BEAM is effective as a bridge to transplantation in patients with refractory or relapsed Hodgkin lymphoma who have failed to respond to previous lines of salvage chemotherapy but not in patients with salvage-refractory DLBCL. Br J Haematol. 2012;157:543–552. - PubMed
-
- Nagle SJ, Woo K, Schuster SJ, et al. Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am J Hematol. 2013;88:890–894. - PubMed
-
- Rivera-Rodriguez N, Cabanillas F. Recent advances in the management of mantle cell lymphoma. Curr Opin Oncol. 2013;25:716–721. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

