Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522
- PMID: 25154822
- PMCID: PMC4162493
- DOI: 10.1200/JCO.2013.53.5633
Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522
Abstract
Purpose: Combining cisplatin or cetuximab with radiation improves overall survival (OS) of patients with stage III or IV head and neck carcinoma (HNC). Cetuximab plus platinum regimens also increase OS in metastatic HNC. The Radiation Therapy Oncology Group launched a phase III trial to test the hypothesis that adding cetuximab to the radiation-cisplatin platform improves progression-free survival (PFS).
Patients and methods: Eligible patients with stage III or IV HNC were randomly assigned to receive radiation and cisplatin without (arm A) or with (arm B) cetuximab. Acute and late reactions were scored using Common Terminology Criteria for Adverse Events (version 3). Outcomes were correlated with patient and tumor features and markers.
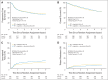
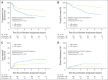
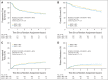
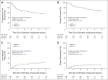
Results: Of 891 analyzed patients, 630 were alive at analysis (median follow-up, 3.8 years). Cetuximab plus cisplatin-radiation, versus cisplatin-radiation alone, resulted in more frequent interruptions in radiation therapy (26.9% v. 15.1%, respectively); similar cisplatin delivery (mean, 185.7 mg/m2 v. 191.1 mg/m2, respectively); and more grade 3 to 4 radiation mucositis (43.2% v. 33.3%, respectively), rash, fatigue, anorexia, and hypokalemia, but not more late toxicity. No differences were found between arms A and B in 30-day mortality (1.8% v. 2.0%, respectively; P = .81), 3-year PFS (61.2% v. 58.9%, respectively; P = .76), 3-year OS (72.9% v. 75.8%, respectively; P = .32), locoregional failure (19.9% v. 25.9%, respectively; P = .97), or distant metastasis (13.0% v. 9.7%, respectively; P = .08). Patients with p16-positive oropharyngeal carcinoma (OPC), compared with patients with p16-negative OPC, had better 3-year probability of PFS (72.8% v. 49.2%, respectively; P < .001) and OS (85.6% v. 60.1%, respectively; P < .001), but tumor epidermal growth factor receptor (EGFR) expression did not distinguish outcome.
Conclusion: Adding cetuximab to radiation-cisplatin did not improve outcome and hence should not be prescribed routinely. PFS and OS were higher in patients with p16-positive OPC, but outcomes did not differ by EGFR expression.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Combining cetuximab with chemoradiotherapy in locally advanced head and neck squamous cell carcinoma: is more better?J Clin Oncol. 2014 Sep 20;32(27):2929-31. doi: 10.1200/JCO.2014.56.1902. J Clin Oncol. 2014. PMID: 25002730 No abstract available.
-
Reply to D. Adkins et al.J Clin Oncol. 2015 Apr 1;33(10):1225-6. doi: 10.1200/JCO.2014.59.9449. Epub 2015 Feb 23. J Clin Oncol. 2015. PMID: 25713435 No abstract available.
-
Reply to D. Adkins et al.J Clin Oncol. 2015 Apr 1;33(10):1224-5. doi: 10.1200/JCO.2014.59.9431. Epub 2015 Feb 23. J Clin Oncol. 2015. PMID: 25713438 No abstract available.
-
RTOG 0522: huge Investment in patients and resources and no benefit with addition of cetuximab to radiotherapy--why did this occur?J Clin Oncol. 2015 Apr 1;33(10):1223-4. doi: 10.1200/JCO.2014.59.6908. Epub 2015 Feb 23. J Clin Oncol. 2015. PMID: 25713440 No abstract available.
References
-
- Pignon JP, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomized trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. - PubMed
-
- Rubin Grandis J, Melhem MF, Barnes EL, et al. Quantitative immunohistochemical analysis of transforming growth factor-alpha and epidermal growth factor receptor in patients with squamous cell carcinoma of the head and neck. Cancer. 1996;78:1284–1292. - PubMed
-
- Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. - PubMed
-
- Psyrri A, Yu Z, Weinberger PM, et al. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res. 2005;11:5856–5862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

