Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation
- PMID: 25154831
- PMCID: PMC4178523
- DOI: 10.1200/JCO.2013.53.8157
Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation
Abstract
Purpose: Age has long been used as a major factor for assessing suitability for allogeneic hematopoietic cell transplantation (HCT). The HCT-comorbidity index (HCT-CI) was developed as a measure of health status to predict mortality risk after HCT. Whether age, comorbidities, or both should guide decision making for HCT is unknown.
Patients and methods: Data from 3,033 consecutive recipients of HLA-matched grafts from five institutions contributed to this analysis. Patients were randomly divided into a training set to develop weights for age intervals and a validation set to assess the performance of prognostic models.
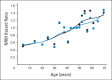
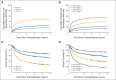
Results: In the training set, patients age 20 to 39 years, 40 to 49 years, 50 to 59 years, and ≥ 60 years had hazard ratios for nonrelapse mortality (NRM) of 1.21 (P = .29), 1.48 (P = .04), 1.75 (P = .004), and 1.84 (P = .005), respectively, compared with those age younger than 20 years. Consequently, age ≥ 40 years was assigned a weight of 1 to be added to the HCT-CI to constitute a composite comorbidity/age index. In the validation set, the composite comorbidity/age score had statistically significantly higher c-statistic estimates for prediction of NRM (0.664 v 0.556; P < .001) and survival (0.682 v 0.560; P < .001) compared with age, respectively. Patients with comorbidity/age scores of 0 to 2 had comparable mortality risks regardless of conditioning regimens. Patients with scores of 3 to 4 and ≥ 5 had statistically significant higher mortality risks after high-dose versus nonmyeloablative regimens.
Conclusion: Age is a poor prognostic factor. The proposed composite measure allows integration of both comorbidities and age into clinical decision making and comparative-effectiveness research of HCT.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Grimwade D, Hills RK. Independent prognostic factors for AML outcome. Hematology Am Soc Hematol Educ Program. 2009;2009:385–395. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

