Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques
- PMID: 25155019
- PMCID: PMC4172223
- DOI: 10.1084/jem.20132494
Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques
Abstract
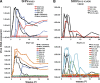
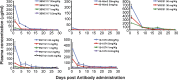
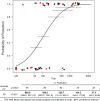
It is widely appreciated that effective human vaccines directed against viral pathogens elicit neutralizing antibodies (NAbs). The passive transfer of anti-HIV-1 NAbs conferring sterilizing immunity to macaques has been used to determine the plasma neutralization titers, which must be present at the time of exposure, to prevent acquisition of SIV/HIV chimeric virus (SHIV) infections. We administered five recently isolated potent and broadly acting anti-HIV neutralizing monoclonal antibodies (mAbs) to rhesus macaques and challenged them intrarectally 24 h later with either of two different R5-tropic SHIVs. By combining the results obtained from 60 challenged animals, we determined that the protective neutralization titer in plasma preventing virus infection in 50% of the exposed monkeys was relatively modest (∼1:100) and potentially achievable by vaccination.
Figures



References
-
- Advisory Committee on Immunization Practices (ACIP), Fiore A.E., Wasley A., and Bell B.P.. 2006. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 55:1–23 - PubMed
-
- Baba, T.W., Liska V., Hofmann-Lehmann R., Vlasak J., Xu W., Ayehunie S., Cavacini L.A., Posner M.R., Katinger H., Stiegler G., et al. . 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200–206 10.1038/72309 - DOI - PubMed
-
- Barnett, S.W., Srivastava I.K., Kan E., Zhou F., Goodsell A., Cristillo A.D., Ferrai M.G., Weiss D.E., Letvin N.L., Montefiori D., et al. . 2008. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS. 22:339–348 10.1097/QAD.0b013e3282f3ca57 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

