Who regenerates the kidney tubule?
- PMID: 25155054
- PMCID: PMC4438740
- DOI: 10.1093/ndt/gfu281
Who regenerates the kidney tubule?
Abstract
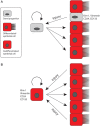
The kidney possesses profound regenerative potential and in some cases can recover completely 'restitutio at integrum' following an acute kidney injury (AKI). Emerging evidence strongly suggests that sometimes repair is incomplete, however, and, in this situation, an episode of AKI leads to future chronic kidney disease (CKD). Understanding the tubular response after AKI will shed light on the relationship between incomplete repair and future risk of CKD. The first repair phase after AKI is characterized by robust proliferation of epithelial cells in the proximal tubule. The exact source of these proliferating cells has been a source of controversy for the last decade. While nearly everyone now agrees that reparative cells arise within the proximal tubule, there is disagreement about whether all surviving cells possess an equivalent repair capacity through dedifferentiation, or alternatively whether a pre-existing intratubular stem cell population [so-called scattered tubular cells (STC)] is responsible for repair. This review will summarize the evidence on both sides of this issue and will discuss very recent genetic fate-tracing data that strongly points against the existence of intratubular stem cells but rather indicates that terminally differentiated proximal tubule epithelial cells undergo dedifferentiation upon injury to replace lost neighboring tubular epithelial cells through proliferative self-duplication. This new evidence includes data clearly indicating that STC are not committed tubular stem cells but instead represent individual dedifferentiated tubular epithelial cells that transiently express putative stem cell markers.
Keywords: acute kidney injury; dedifferentiation; stem cell; tubular repair.
© The Author 2014. Published by Oxford University Press on behalf of ERA-EDTA. All rights reserved.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

