CD38 expression in early B-cell precursors contributes to extracellular signal-regulated kinase-mediated apoptosis
- PMID: 25155483
- PMCID: PMC4298421
- DOI: 10.1111/imm.12370
CD38 expression in early B-cell precursors contributes to extracellular signal-regulated kinase-mediated apoptosis
Abstract
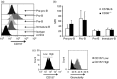
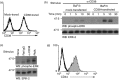
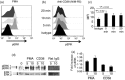
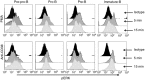
CD38 is a 45,000 molecular weight transmembrane protein that is expressed in immature and mature lymphocytes. However, the expression and function of CD38 during B-cell differentiation in mice is poorly understood. Here, we report that CD38 is expressed from the earliest stages of B-cell development. Pre-pro-B, pro-B, pre-B and immature B cells from murine bone marrow all stained positive for CD38. Interestingly, CD38 expression increases with B-cell maturation. To assess the role of CD38 during B-cell maturation, CD38-deficient mice were analysed. CD38(-/-) mice showed a significant increase in both the frequency of B-lineage cells and the absolute numbers of pre-pro-B cells in bone marrow; however, no other differences were observed at later stages. CD38 cross-linking in Ba/F3 cells promoted apoptosis and marked extracellular signal-regulated kinase (ERK) phosphorylation, and these effects were reduced by treatment with the mitogen-activated protein kinase/ERK kinase inhibitor PD98059, and similar effects were observed in B-cell precursors from bone marrow. These data demonstrate that B-cell precursors in mouse bone marrow express functional CD38 and implicate the early ligation of CD38 in the ERK-associated regulation of the B-lineage differentiation pathway.
Keywords: CD38; bone marrow; extracellular signal-regulated kinase; mouse B-cell development.
© 2014 John Wiley & Sons Ltd.
Figures




Similar articles
-
CD38 induces differentiation of immature transitional 2 B lymphocytes in the spleen.Blood. 2008 Apr 1;111(7):3644-52. doi: 10.1182/blood-2007-08-107714. Epub 2008 Jan 25. Blood. 2008. PMID: 18223169
-
CD38 cleavage in fMLP- and IL-8-induced chemotaxis is dependent on p38 MAP kinase but independent of p44/42 MAP kinase.Cell Signal. 2005 Feb;17(2):167-75. doi: 10.1016/j.cellsig.2004.06.008. Cell Signal. 2005. PMID: 15494208
-
CD38 Deficiency Downregulates the Onset and Pathogenesis of Collagen-Induced Arthritis through the NF-κB Pathway.J Immunol Res. 2019 Mar 5;2019:7026067. doi: 10.1155/2019/7026067. eCollection 2019. J Immunol Res. 2019. PMID: 30949517 Free PMC article.
-
CD38: a new paradigm in lymphocyte activation and signal transduction.Immunol Rev. 1998 Feb;161:79-93. doi: 10.1111/j.1600-065x.1998.tb01573.x. Immunol Rev. 1998. PMID: 9553766 Review.
-
Innate immunity is regulated by CD38, an ecto-enzyme with ADP-ribosyl cyclase activity.Microbes Infect. 2003 Jan;5(1):49-58. doi: 10.1016/s1286-4579(02)00055-2. Microbes Infect. 2003. PMID: 12593973 Review.
Cited by
-
Ectonucleotidases in Blood Malignancies: A Tale of Surface Markers and Therapeutic Targets.Front Immunol. 2019 Oct 4;10:2301. doi: 10.3389/fimmu.2019.02301. eCollection 2019. Front Immunol. 2019. PMID: 31636635 Free PMC article. Review.
-
Chemical modulation of apoptosis in molluscan cell cultures.Cell Stress Chaperones. 2019 Sep;24(5):905-916. doi: 10.1007/s12192-019-01014-x. Epub 2019 Jun 22. Cell Stress Chaperones. 2019. PMID: 31230213 Free PMC article.
-
Detection of Human CD38 Using Variable Lymphocyte Receptor (VLR) Tetramers.Cells. 2020 Apr 12;9(4):950. doi: 10.3390/cells9040950. Cells. 2020. PMID: 32290546 Free PMC article.
-
CAR T-cell therapy in autoimmune diseases: a promising frontier on the horizon.Front Immunol. 2025 Aug 12;16:1613878. doi: 10.3389/fimmu.2025.1613878. eCollection 2025. Front Immunol. 2025. PMID: 40873568 Free PMC article. Review.
References
-
- Zocchi E, Franco L, Guida L, Benatti U, Bargellesi A, Malavasi F, Lee HC, De Flora A. A single protein immunologically identified as CD38 displays NAD– glycohydrolase, ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities at the outer surface of human erythrocytes. Biochem Biophys Res Commn. 1993;196:1459–65. - PubMed
-
- Santos-Argumedo L, Teixeira C, Perece G, Kirkham P, Parkhouse RME. A B lymphocyte surface molecule mediating activation and protection from apoptosis via calcium channels. J Immunol. 1993;151:3119–30. - PubMed
-
- Lund FE, Solvanson N, Cooke MP, Health AW, Grimaldi JC, Parkhouse RM, Goodnow CC, Howard MC. Signaling through murine CD38 is impaired in antigen receptor-unresponsive B cells. Eur J Immunol. 1995;25:1338–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

