Actin is required for IFT regulation in Chlamydomonas reinhardtii
- PMID: 25155506
- PMCID: PMC4160380
- DOI: 10.1016/j.cub.2014.07.038
Actin is required for IFT regulation in Chlamydomonas reinhardtii
Abstract
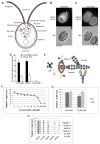
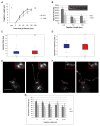
Assembly of cilia and flagella requires intraflagellar transport (IFT), a highly regulated kinesin-based transport system that moves cargo from the basal body to the tip of flagella [1]. The recruitment of IFT components to basal bodies is a function of flagellar length, with increased recruitment in rapidly growing short flagella [2]. The molecular pathways regulating IFT are largely a mystery. Because actin network disruption leads to changes in ciliary length and number, actin has been proposed to have a role in ciliary assembly. However, the mechanisms involved are unknown. In Chlamydomonas reinhardtii, conventional actin is found in both the cell body and the inner dynein arm complexes within flagella [3, 4]. Previous work showed that treating Chlamydomonas cells with the actin-depolymerizing compound cytochalasin D resulted in reversible flagellar shortening [5], but how actin is related to flagellar length or assembly remains unknown. Here we utilize small-molecule inhibitors and genetic mutants to analyze the role of actin dynamics in flagellar assembly in Chlamydomonas reinhardtii. We demonstrate that actin plays a role in IFT recruitment to basal bodies during flagellar elongation and that when actin is perturbed, the normal dependence of IFT recruitment on flagellar length is lost. We also find that actin is required for sufficient entry of IFT material into flagella during assembly. These same effects are recapitulated with a myosin inhibitor, suggesting that actin may act via myosin in a pathway by which flagellar assembly is regulated by flagellar length.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Cilia assembly: a role for F-actin in IFT recruitment.Curr Biol. 2014 Sep 8;24(17):R796-8. doi: 10.1016/j.cub.2014.07.043. Curr Biol. 2014. PMID: 25202869
References
-
- Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. - PubMed
-
- Kato-Minoura T, Uryu S, Hirono M, Kamiya R. Highly divergent actin expressed in a Chlamydomonas mutant lacking the conventional actin gene. Biochem Biophys Res Commun. 1998;251:71–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

