Hydrogels and scaffolds for immunomodulation
- PMID: 25155610
- PMCID: PMC4269549
- DOI: 10.1002/adma.201402105
Hydrogels and scaffolds for immunomodulation
Abstract
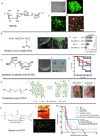
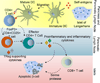
For over two decades, immunologists and biomaterials scientists have co-existed in parallel world with the rationale of understanding the molecular profile of immune responses to vaccination, implantation, and treating incurable diseases. Much of the field of biomaterial-based immunotherapy has relied on evaluating model antigens such as chicken egg ovalbumin in mouse models but their relevance to humans has been point of much discussion. Nevertheless, such model antigens have provided important insights into the mechanisms of immune regulation and served as a proof-of-concept for plethora of biomaterial-based vaccines. After years of extensive development of numerous biomaterials for immunomodulation, it is only recently that an experimental scaffold vaccine implanted beneath the skin has begun to use the human model to study the immune responses to cancer vaccination by co-delivering patient-derived tumor lysates and immunomodulatory proteins. If successful, this scaffold vaccine will change the way we approached untreatable cancers, but more importantly, will allow a faster and more rational translation of therapeutic regimes to other cancers, chronic infections, and autoimmune diseases. Most materials reviews have focused on immunomodulatory adjuvants and micro-nano-particles. Here we provide an insight into emerging hydrogel and scaffold based immunomodulatory approaches that continue to demonstrate efficacy against immune associated diseases.
Keywords: biomaterials; hydrogels; immunology; immunomodulation; polymers; scaffolds.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- Finn OJ. Nat Rev Immunol. 2003;3:630. - PubMed
-
- Flaherty KT, Hodi FS, Fisher DE. Nat Rev Cancer. 2012;12:349. - PubMed
-
- Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. Nat Rev Cancer. 2008;8:11. - PubMed
-
- Monsalvo AC, Batalle JP, Lopez MF, Krause JC, Klemenc J, Hernandez JZ, Maskin B, Bugna J, Rubinstein C, Aguilar L, Dalurzo L, Libster R, Savy V, Baumeister E, Cabral G, Font J, Solari L, Weller KP, Johnson J, Echavarria M, Edwards KM, Chappell JD, Crowe JE, Jr., Williams JV, Melendi GA, Polack FP. Nature medicine. 2011;17:195. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

