Combining viral vectored and protein-in-adjuvant vaccines against the blood-stage malaria antigen AMA1: report on a phase 1a clinical trial
- PMID: 25156127
- PMCID: PMC4250079
- DOI: 10.1038/mt.2014.157
Combining viral vectored and protein-in-adjuvant vaccines against the blood-stage malaria antigen AMA1: report on a phase 1a clinical trial
Abstract
The development of effective vaccines against difficult disease targets will require the identification of new subunit vaccination strategies that can induce and maintain effective immune responses in humans. Here we report on a phase 1a clinical trial using the AMA1 antigen from the blood-stage Plasmodium falciparum malaria parasite delivered either as recombinant protein formulated with Alhydrogel adjuvant with and without CPG 7909, or using recombinant vectored vaccines--chimpanzee adenovirus ChAd63 and the orthopoxvirus MVA. A variety of promising "mixed-modality" regimens were tested. All volunteers were primed with ChAd63, and then subsequently boosted with MVA and/or protein-in-adjuvant using either an 8- or 16-week prime-boost interval. We report on the safety of these regimens, as well as the T cell, B cell, and serum antibody responses. Notably, IgG antibody responses primed by ChAd63 were comparably boosted by AMA1 protein vaccine, irrespective of whether CPG 7909 was included in the Alhydrogel adjuvant. The ability to improve the potency of a relatively weak aluminium-based adjuvant in humans, by previously priming with an adenoviral vaccine vector encoding the same antigen, thus offers a novel vaccination strategy for difficult or neglected disease targets when access to more potent adjuvants is not possible.
Figures
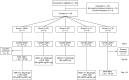

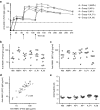
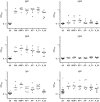

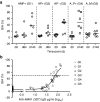
References
-
- World Health Organization World Malaria Report 2012. World Malaria Report. 2012;2012:1–249.
-
- Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. - PubMed
-
- Birkett AJ, Moorthy VS, Loucq C, Chitnis CE, Kaslow DC. Malaria vaccine R&D in the Decade of Vaccines: breakthroughs, challenges and opportunities. Vaccine. 2013;31 suppl. 2:B233–B243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

