Protective effects of farnesoid X receptor (FXR) on hepatic lipid accumulation are mediated by hepatic FXR and independent of intestinal FGF15 signal
- PMID: 25156247
- PMCID: PMC4146754
- DOI: 10.1111/liv.12456
Protective effects of farnesoid X receptor (FXR) on hepatic lipid accumulation are mediated by hepatic FXR and independent of intestinal FGF15 signal
Abstract
Background & aims: There is a growing evidence that bile acids are involved in the regulation of triglyceride-, cholesterol-homoeostasis and fat absorption. In this study organ-specific Fxr knockout mice were used to further investigate the influence of farnesoid X receptor FXR in lipogenesis.
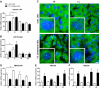
Methods: Liver- and intestine-specific Fxr knockout mice were fed a 1% cholesterol diet for 28 days. Histological examination of frozen tissue sections included Sudan III/H&E, BODIPY staining and liver X receptor (LXR) immunohistochemistry. Liver triglycerides, serum cholesterol, serum bile acids and nuclear LXR protein were measured. mRNA expression of several genes involved in bile acid-, cholesterol-homoeostasis and lipogenesis was quantified by real-time PCR.
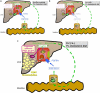
Results: Hepatic FXR deficiency contributes to lipid accumulation under 1% cholesterol administration which is not observed in intestinal Fxr knockout mice. Strong lipid accumulation, characterized by larger vacuoles could be observed in hepatic Fxr knockout sections, while intestinal Fxr knockout mice show no histological difference to controls. In addition, these mice have the ability to maintain normal serum cholesterol and bile acid levels. Hepatic Fxr knockouts were characterized by elevated triglycerides and bile acid levels. Expression level of LXR was significantly elevated under control and 1% cholesterol diet in hepatic Fxr knockout mice and was followed by concomitant lipogenic target gene induction such as Fas and Scd-1. This protective FXR effect against hepatic lipid accumulation was independent of intestinal Fgf15 induction.
Conclusion: These results show that the principal site of protective bile acid signalling against lipid accumulation is located in the liver since the absence of hepatic but not intestinal FXR contributes to lipid accumulation under cholesterol diet.
Keywords: FXR; NAFLD; bile acids; lipogenesis.
© 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures

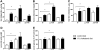

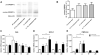
Similar articles
-
Intestinal FXR-mediated FGF15 production contributes to diurnal control of hepatic bile acid synthesis in mice.Lab Invest. 2010 Oct;90(10):1457-67. doi: 10.1038/labinvest.2010.107. Epub 2010 Jun 7. Lab Invest. 2010. PMID: 20531290 Free PMC article.
-
Schaftoside alleviates HFD-induced hepatic lipid accumulation in mice via upregulating farnesoid X receptor.J Ethnopharmacol. 2020 Jun 12;255:112776. doi: 10.1016/j.jep.2020.112776. Epub 2020 Mar 20. J Ethnopharmacol. 2020. PMID: 32205261
-
Yangonin protects against non-alcoholic fatty liver disease through farnesoid X receptor.Phytomedicine. 2019 Feb;53:134-142. doi: 10.1016/j.phymed.2018.09.006. Epub 2018 Sep 5. Phytomedicine. 2019. PMID: 30668392
-
Nuclear receptors and cholesterol metabolism in the intestine.Atheroscler Suppl. 2015 Feb;17:9-11. doi: 10.1016/S1567-5688(15)50003-2. Atheroscler Suppl. 2015. PMID: 25659870 Review.
-
FXR an emerging therapeutic target for the treatment of atherosclerosis.J Cell Mol Med. 2010 Jan;14(1-2):79-92. doi: 10.1111/j.1582-4934.2009.00997.x. J Cell Mol Med. 2010. PMID: 20041971 Free PMC article. Review.
Cited by
-
Why Bile Acids Are So Important in Non-Alcoholic Fatty Liver Disease (NAFLD) Progression.Cells. 2019 Oct 30;8(11):1358. doi: 10.3390/cells8111358. Cells. 2019. PMID: 31671697 Free PMC article. Review.
-
Enteric Microbiota⁻Gut⁻Brain Axis from the Perspective of Nuclear Receptors.Int J Mol Sci. 2018 Jul 28;19(8):2210. doi: 10.3390/ijms19082210. Int J Mol Sci. 2018. PMID: 30060580 Free PMC article. Review.
-
Grape Seed Procyanidins and Cholestyramine Differentially Alter Bile Acid and Cholesterol Homeostatic Gene Expression in Mouse Intestine and Liver.PLoS One. 2016 Apr 25;11(4):e0154305. doi: 10.1371/journal.pone.0154305. eCollection 2016. PLoS One. 2016. PMID: 27111442 Free PMC article.
-
Present and Future: Crosstalks Between Polycystic Ovary Syndrome and Gut Metabolites Relating to Gut Microbiota.Front Endocrinol (Lausanne). 2022 Jul 19;13:933110. doi: 10.3389/fendo.2022.933110. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35928893 Free PMC article. Review.
-
Bile acids at the cross-roads of gut microbiome-host cardiometabolic interactions.Diabetol Metab Syndr. 2017 Dec 28;9:102. doi: 10.1186/s13098-017-0299-9. eCollection 2017. Diabetol Metab Syndr. 2017. PMID: 29299069 Free PMC article. Review.
References
-
- Mendez-Sanchez N, Arrese M, Zamora-Valdes D, Uribe M. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int. 2007;27:423–433. - PubMed
-
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. - PubMed
-
- Stefan N, Kantartzis K, Haring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29:939–960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

