Unconventionally secreted effectors of two filamentous pathogens target plant salicylate biosynthesis
- PMID: 25156390
- PMCID: PMC4348438
- DOI: 10.1038/ncomms5686
Unconventionally secreted effectors of two filamentous pathogens target plant salicylate biosynthesis
Abstract
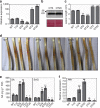
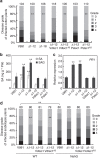
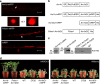
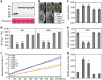
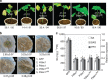
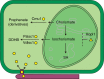
Plant diseases caused by fungi and oomycetes pose an increasing threat to food security and ecosystem health worldwide. These filamentous pathogens, while taxonomically distinct, modulate host defense responses by secreting effectors, which are typically identified based on the presence of signal peptides. Here we show that Phytophthora sojae and Verticillium dahliae secrete isochorismatases (PsIsc1 and VdIsc1, respectively) that are required for full pathogenesis. PsIsc1 and VdIsc1 can suppress salicylate-mediated innate immunity in planta and hydrolyse isochorismate in vitro. A conserved triad of catalytic residues is essential for both functions. Thus, the two proteins are isochorismatase effectors that disrupt the plant salicylate metabolism pathway by suppressing its precursor. Furthermore, these proteins lack signal peptides, but exhibit characteristics that lead to unconventional secretion. Therefore, this secretion pathway is a novel mechanism for delivering effectors and might play an important role in host-pathogen interactions.
Figures

References
-
- Tyler B. M. et al. Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science 313, 1261–1266 (2006). - PubMed
-
- Kamoun S. A catalogue of the effector secretome of plant pathogenic oomycetes. Annu. Rev. Phytopathol. 44, 41–60 (2006). - PubMed
-
- Richards T. A. et al. Evolution of filamentous plant pathogens: gene exchange across eukaryotic kingdoms. Curr. Biol. 16, 1857–1864 (2006). - PubMed
-
- Jones J. D. & Dangl J. L. The plant immune system. Nature 444, 323–329 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

