Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro wound model
- PMID: 25156721
- PMCID: PMC4249327
- DOI: 10.1128/IAI.02198-14
Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro wound model
Abstract
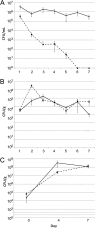
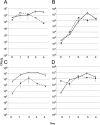
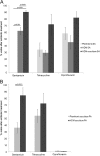
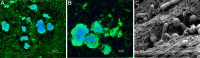
In individuals with polymicrobial infections, microbes often display synergistic interactions that can enhance their colonization, virulence, or persistence. One of the most prevalent types of polymicrobial infection occurs in chronic wounds, where Pseudomonas aeruginosa and Staphylococcus aureus are the two most common causes. Although they are the most commonly associated microbial species in wound infections, very little is known about their interspecies relationship. Evidence suggests that P. aeruginosa-S. aureus coinfections are more virulent than monoculture infection with either species; however, difficulties in growing these two pathogens together in vitro have hampered attempts to uncover the mechanisms involved. Here we describe a simple and clinically relevant in vitro wound model that supported concomitant growth of P. aeruginosa and S. aureus. We observed that the ability of P. aeruginosa and S. aureus to survive antibiotic treatment increased when they were grown together in planktonic cocultures and that antibiotic tolerance was further enhanced when they were grown together in the wound model. We attributed this enhanced tolerance to both the "host-derived" and "bacterium-derived" matrix components. Taken together, our data indicate that P. aeruginosa and S. aureus may benefit each other by coinfecting wounds and that the host-derived matrix may serve as important a role as the bacterium-derived matrix in protecting bacteria from some antibiotics.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Cunningham AB. 2006. Biofilms: the hypertextbook. Montana State University, Bozeman, MT.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

