Influenza A virus alters pneumococcal nasal colonization and middle ear infection independently of phase variation
- PMID: 25156728
- PMCID: PMC4249341
- DOI: 10.1128/IAI.01856-14
Influenza A virus alters pneumococcal nasal colonization and middle ear infection independently of phase variation
Abstract
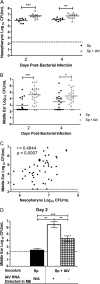
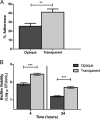
Streptococcus pneumoniae (pneumococcus) is both a widespread nasal colonizer and a leading cause of otitis media, one of the most common diseases of childhood. Pneumococcal phase variation influences both colonization and disease and thus has been linked to the bacteria's transition from colonizer to otopathogen. Further contributing to this transition, coinfection with influenza A virus has been strongly associated epidemiologically with the dissemination of pneumococci from the nasopharynx to the middle ear. Using a mouse infection model, we demonstrated that coinfection with influenza virus and pneumococci enhanced both colonization and inflammatory responses within the nasopharynx and middle ear chamber. Coinfection studies were also performed using pneumococcal populations enriched for opaque or transparent phase variants. As shown previously, opaque variants were less able to colonize the nasopharynx. In vitro, this phase also demonstrated diminished biofilm viability and epithelial adherence. However, coinfection with influenza virus ameliorated this colonization defect in vivo. Further, viral coinfection ultimately induced a similar magnitude of middle ear infection by both phase variants. These data indicate that despite inherent differences in colonization, the influenza A virus exacerbation of experimental middle ear infection is independent of the pneumococcal phase. These findings provide new insights into the synergistic link between pneumococcus and influenza virus in the context of otitis media.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

