Localization of Burkholderia cepacia complex bacteria in cystic fibrosis lungs and interactions with Pseudomonas aeruginosa in hypoxic mucus
- PMID: 25156735
- PMCID: PMC4249344
- DOI: 10.1128/IAI.01876-14
Localization of Burkholderia cepacia complex bacteria in cystic fibrosis lungs and interactions with Pseudomonas aeruginosa in hypoxic mucus
Abstract
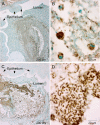
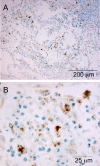
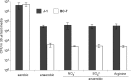
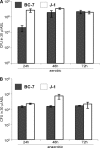
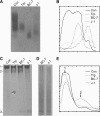
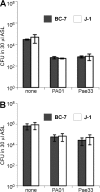
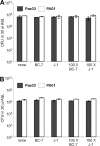
The localization of Burkholderia cepacia complex (Bcc) bacteria in cystic fibrosis (CF) lungs, alone or during coinfection with Pseudomonas aeruginosa, is poorly understood. We performed immunohistochemistry for Bcc and P. aeruginosa bacteria on 21 coinfected or singly infected CF lungs obtained at transplantation or autopsy. Parallel in vitro experiments examined the growth of two Bcc species, Burkholderia cenocepacia and Burkholderia multivorans, in environments similar to those occupied by P. aeruginosa in the CF lung. Bcc bacteria were predominantly identified in the CF lung as single cells or small clusters within phagocytes and mucus but not as "biofilm-like structures." In contrast, P. aeruginosa was identified in biofilm-like masses, but densities appeared to be reduced during coinfection with Bcc bacteria. Based on chemical analyses of CF and non-CF respiratory secretions, a test medium was defined to study Bcc growth and interactions with P. aeruginosa in an environment mimicking the CF lung. When test medium was supplemented with alternative electron acceptors under anaerobic conditions, B. cenocepacia and B. multivorans used fermentation rather than anaerobic respiration to gain energy, consistent with the identification of fermentation products by high-performance liquid chromatography (HPLC). Both Bcc species also expressed mucinases that produced carbon sources from mucins for growth. In the presence of P. aeruginosa in vitro, both Bcc species grew anaerobically but not aerobically. We propose that Bcc bacteria (i) invade a P. aeruginosa-infected CF lung when the airway lumen is anaerobic, (ii) inhibit P. aeruginosa biofilm-like growth, and (iii) expand the host bacterial niche from mucus to also include macrophages.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Cystic Fibrosis Foundation. 2013. Cystic Fibrosis Foundation patient registry: 2012 annual data report. Cystic Fibrosis Foundation, Bethesda, MD.
-
- Frangolias DD, Mahenthiralingam E, Rae S, Raboud JM, Davidson AGF, Wittmann R, Wilcox PG. 1999. Burkholderia cepacia in cystic fibrosis—variable disease course. Am. J. Respir. Crit. Care Med. 160:1572–1577. - PubMed
-
- Riedel K, Hentzer M, Geisenberger O, Huber B, Steidle A, Wu H, Høiby N, Givskov M, Molin S, Eberl L. 2001. N-Acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology 147:3249–3262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

