Dose escalation and dosimetry of first-in-human α radioimmunotherapy with 212Pb-TCMC-trastuzumab
- PMID: 25157044
- PMCID: PMC4486012
- DOI: 10.2967/jnumed.114.143842
Dose escalation and dosimetry of first-in-human α radioimmunotherapy with 212Pb-TCMC-trastuzumab
Abstract
Our purpose was to study the safety, distribution, pharmacokinetics, immunogenicity, and tumor response of intraperitoneal (212)Pb-TCMC-trastuzumab (TCMC is S-2-(4-isothiocyanatobenzyl)-1,4,7,10-tetraaza-1,4,7,10-tetra(2-carbamoylmethyl)cyclododecane) in patients with human epidermal growth factor receptor type 2 (HER-2)-expressing malignancy.
Methods: In a standard 3 + 3 phase 1 design for dose escalation, (212)Pb-TCMC-trastuzumab was delivered intraperitoneally less than 4 h after administration of trastuzumab (4 mg/kg intravenously) to patients with peritoneal carcinomatosis who had failed standard therapies.
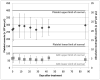
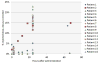
Results: Five dosage levels (7.4, 9.6, 12.6, 16.3, and 21.1 MBq/m(2)) showed minimal toxicity at more than 1 y for the first group and more than 4 mo for others. The lack of substantial toxicity was consistent with the dosimetry assessments (mean equivalent dose to marrow, 0.18 mSv/MBq). Radiation dosimetry assessment was performed using pharmacokinetics data obtained in the initial cohort (n = 3). Limited redistribution of radioactivity out of the peritoneal cavity to circulating blood, which cleared via urinary excretion, and no specific uptake in major organs were observed in 24 h. Maximum serum concentration of the radiolabeled antibody was 22.9% at 24 h (decay-corrected to injection time) and 500 Bq/mL (decay-corrected to collection time). Non-decay-corrected cumulative urinary excretion was 6% or less in 24 h (2.3 half-lives). Dose rate measurements performed at 1 m from the patient registered less than 5μSv/h (using portable detectors) in the latest cohort, significantly less than what is normally observed using nuclear medicine imaging agents. Antidrug antibody assays performed on serum from the first 4 cohorts were all negative.
Conclusion: Five dose levels of intraperitoneal (212)Pb-TCMC-trastuzumab treatment of patients with peritoneal carcinomatosis showed little agent-related toxicity, consistent with the dosimetry calculations.
Keywords: 212Pb-TCMC-trastuzumab; alpha; dosimetry; radioimmunotherapy; radionuclide.
© 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
Conflict of interest statement
No Conflict of Interest for: Ruby Meredith, Sui Shen, Patty Bunch, Desiree Morgan, J. Michael Straughn Jr. or Jinda Fan. Darrell Fisher has served on the scientific Advisory Board; Eileen Banaga and Julien Torgue are employed by the sponsor of the clinical trial (AREVA Med LLC, Bethesda, MD.
Figures
Similar articles
-
Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients.Cancer Biother Radiopharm. 2014 Feb;29(1):12-7. doi: 10.1089/cbr.2013.1531. Epub 2013 Nov 14. Cancer Biother Radiopharm. 2014. PMID: 24229395 Free PMC article. Clinical Trial.
-
Safety and Outcome Measures of First-in-Human Intraperitoneal α Radioimmunotherapy With 212Pb-TCMC-Trastuzumab.Am J Clin Oncol. 2018 Jul;41(7):716-721. doi: 10.1097/COC.0000000000000353. Am J Clin Oncol. 2018. PMID: 27906723 Free PMC article.
-
Gene expression profiling upon (212) Pb-TCMC-trastuzumab treatment in the LS-174T i.p. xenograft model.Cancer Med. 2013 Oct;2(5):646-53. doi: 10.1002/cam4.132. Epub 2013 Sep 19. Cancer Med. 2013. PMID: 24403230 Free PMC article.
-
Experimental radioimmunotherapy.Med Phys. 1993 Mar-Apr;20(2 Pt 2):551-67. doi: 10.1118/1.597142. Med Phys. 1993. PMID: 8492764 Review.
-
Dosimetry of intraperitoneally administered radiolabeled antibodies.Med Phys. 1993 Mar-Apr;20(2 Pt 2):593-600. doi: 10.1118/1.597054. Med Phys. 1993. PMID: 8492768 Review.
Cited by
-
Mechanisms of Cell Killing Response from Low Linear Energy Transfer (LET) Radiation Originating from (177)Lu Radioimmunotherapy Targeting Disseminated Intraperitoneal Tumor Xenografts.Int J Mol Sci. 2016 May 16;17(5):736. doi: 10.3390/ijms17050736. Int J Mol Sci. 2016. PMID: 27196891 Free PMC article.
-
Primary standardization of 212Pb activity by liquid scintillation counting.Appl Radiat Isot. 2022 Dec;190:110473. doi: 10.1016/j.apradiso.2022.110473. Epub 2022 Sep 22. Appl Radiat Isot. 2022. PMID: 36201936 Free PMC article.
-
Radioimmunotherapy in Oncology: Overview of the Last Decade Clinical Trials.Cancers (Basel). 2021 Nov 7;13(21):5570. doi: 10.3390/cancers13215570. Cancers (Basel). 2021. PMID: 34771732 Free PMC article. Review.
-
Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The "Hopeful Eight".Pharmaceutics. 2021 Jun 18;13(6):906. doi: 10.3390/pharmaceutics13060906. Pharmaceutics. 2021. PMID: 34207408 Free PMC article. Review.
-
B7-H3-targeted 212Pb radioimmunotherapy of ovarian cancer in preclinical models.Nucl Med Biol. 2017 Apr;47:23-30. doi: 10.1016/j.nucmedbio.2017.01.003. Epub 2017 Jan 10. Nucl Med Biol. 2017. PMID: 28104527 Free PMC article.
References
-
- Aarts F, Bleichrodt RP, Oyen WJ, Boerman OC. Intracavitary radioimmunotherapy to treat solid tumors. Cancer Biother Radiopharm. 2008 Feb;23:92–107. - PubMed
-
- Epenetos AA, Hird V, Lambert H, Mason P, Coulter C. Long term survival of patients with advanced ovarian cancer treated with intraperitoneal radioimmunotherapy. Int J Gynecol Cancer. 2000 Jan;10:44–46. - PubMed
-
- Oei AL, Verheijen RH, Seiden MV, Benigno BB, Lopes A, Soper JT. Decreased intraperitoneal disease recurrence in epithelial ovarian cancer patients receiving intraperitoneal consolidation treatment with yttrium-90-labeled murine Hmfg1 without improvement in overall survival. IntJCancer. 2007;120:2710–2714. - PubMed
-
- Vergote IB, Vergote-De Vos LN, Abeler VM, et al. Randomized trial comparing cisplatin with radioactive phosphorus or whole-abdomen irradiation as adjuvant treatment of ovarian cancer. Cancer. 1992 Feb 1;69:741–749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
