Listeria monocytogenes is resistant to lysozyme through the regulation, not the acquisition, of cell wall-modifying enzymes
- PMID: 25157076
- PMCID: PMC4248804
- DOI: 10.1128/JB.02053-14
Listeria monocytogenes is resistant to lysozyme through the regulation, not the acquisition, of cell wall-modifying enzymes
Abstract
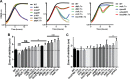
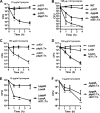
Listeria monocytogenes is a Gram-positive facultative intracellular pathogen that is highly resistant to lysozyme, a ubiquitous enzyme of the innate immune system that degrades cell wall peptidoglycan. Two peptidoglycan-modifying enzymes, PgdA and OatA, confer lysozyme resistance on L. monocytogenes; however, these enzymes are also conserved among lysozyme-sensitive nonpathogens. We sought to identify additional factors responsible for lysozyme resistance in L. monocytogenes. A forward genetic screen for lysozyme-sensitive mutants led to the identification of 174 transposon insertion mutations that mapped to 13 individual genes. Four mutants were killed exclusively by lysozyme and not other cell wall-targeting molecules, including the peptidoglycan deacetylase encoded by pgdA, the putative carboxypeptidase encoded by pbpX, the orphan response regulator encoded by degU, and the highly abundant noncoding RNA encoded by rli31. Both degU and rli31 mutants had reduced expression of pbpX and pgdA, yet DegU and Rli31 did not regulate each other. Since pbpX and pgdA are also present in lysozyme-sensitive bacteria, this suggested that the acquisition of novel enzymes was not responsible for lysozyme resistance, but rather, the regulation of conserved enzymes by DegU and Rli31 conferred high lysozyme resistance. Each lysozyme-sensitive mutant exhibited attenuated virulence in mice, and a time course of infection revealed that the most lysozyme-sensitive strain was killed within 30 min of intravenous infection, a phenotype that was recapitulated in purified blood. Collectively, these data indicate that the genes required for lysozyme resistance are highly upregulated determinants of L. monocytogenes pathogenesis that are required for avoiding the enzymatic activity of lysozyme in the blood.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Stimulation of PgdA-dependent peptidoglycan N-deacetylation by GpsB-PBP A1 in Listeria monocytogenes.Mol Microbiol. 2018 Feb;107(4):472-487. doi: 10.1111/mmi.13893. Epub 2017 Dec 22. Mol Microbiol. 2018. PMID: 29215169
-
EslB Is Required for Cell Wall Biosynthesis and Modification in Listeria monocytogenes.J Bacteriol. 2021 Jan 25;203(4):e00553-20. doi: 10.1128/JB.00553-20. Print 2021 Jan 25. J Bacteriol. 2021. PMID: 33229460 Free PMC article.
-
SpoVG Is a Conserved RNA-Binding Protein That Regulates Listeria monocytogenes Lysozyme Resistance, Virulence, and Swarming Motility.mBio. 2016 Apr 5;7(2):e00240. doi: 10.1128/mBio.00240-16. mBio. 2016. PMID: 27048798 Free PMC article.
-
Analysis of the peptidoglycan hydrolases of Listeria monocytogenes: multiple enzymes with multiple functions.Pol J Microbiol. 2004;53 Suppl:29-34. Pol J Microbiol. 2004. PMID: 15787194 Review.
-
The intrinsic cephalosporin resistome of Listeria monocytogenes in the context of stress response, gene regulation, pathogenesis and therapeutics.J Appl Microbiol. 2016 Feb;120(2):251-65. doi: 10.1111/jam.12989. Epub 2015 Dec 28. J Appl Microbiol. 2016. PMID: 26509460 Review.
Cited by
-
Muropeptides and muropeptide transporters impact on host immune response.Gut Microbes. 2024 Jan-Dec;16(1):2418412. doi: 10.1080/19490976.2024.2418412. Epub 2024 Oct 22. Gut Microbes. 2024. PMID: 39439228 Free PMC article. Review.
-
Cell Shape and Antibiotic Resistance Are Maintained by the Activity of Multiple FtsW and RodA Enzymes in Listeria monocytogenes.mBio. 2019 Aug 6;10(4):e01448-19. doi: 10.1128/mBio.01448-19. mBio. 2019. PMID: 31387909 Free PMC article.
-
Microbiological Quality of Raw Donkey Milk from Serbia and Its Antibacterial Properties at Pre-Cooling Temperature.Animals (Basel). 2023 Jan 17;13(3):327. doi: 10.3390/ani13030327. Animals (Basel). 2023. PMID: 36766215 Free PMC article.
-
Listeria monocytogenes GlmR Is an Accessory Uridyltransferase Essential for Cytosolic Survival and Virulence.mBio. 2023 Apr 25;14(2):e0007323. doi: 10.1128/mbio.00073-23. Epub 2023 Mar 20. mBio. 2023. PMID: 36939339 Free PMC article.
-
Detoxification of methylglyoxal by the glyoxalase system is required for glutathione availability and virulence activation in Listeria monocytogenes.PLoS Pathog. 2021 Aug 18;17(8):e1009819. doi: 10.1371/journal.ppat.1009819. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34407151 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

