Holins in bacteria, eukaryotes, and archaea: multifunctional xenologues with potential biotechnological and biomedical applications
- PMID: 25157079
- PMCID: PMC4288690
- DOI: 10.1128/JB.02046-14
Holins in bacteria, eukaryotes, and archaea: multifunctional xenologues with potential biotechnological and biomedical applications
Abstract
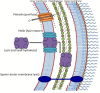
Holins form pores in the cytoplasmic membranes of bacteria for the primary purpose of releasing endolysins that hydrolyze the cell wall and induce cell death. Holins are encoded within bacteriophage genomes, where they promote cell lysis for virion release, and within bacterial genomes, where they serve a diversity of potential or established functions. These include (i) release of gene transfer agents, (ii) facilitation of programs of differentiation such as those that allow sporulation and spore germination, (iii) contribution to biofilm formation, (iv) promotion of responses to stress conditions, and (v) release of toxins and other proteins. There are currently 58 recognized families of holins and putative holins with members exhibiting between 1 and 4 transmembrane α-helical spanners, but many more families have yet to be discovered. Programmed cell death in animals involves holin-like proteins such as Bax and Bak that may have evolved from bacterial holins. Holin homologues have also been identified in archaea, suggesting that these proteins are ubiquitous throughout the three domains of life. Phage-mediated cell lysis of dual-membrane Gram-negative bacteria also depends on outer membrane-disrupting "spanins" that function independently of, but in conjunction with, holins and endolysins. In this minireview, we provide an overview of their modes of action and the first comprehensive summary of the many currently recognized and postulated functions and uses of these cell lysis systems. It is anticipated that future studies will result in the elucidation of many more such functions and the development of additional applications.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

