Mycoplasma pneumoniae, an underutilized model for bacterial cell biology
- PMID: 25157081
- PMCID: PMC4248795
- DOI: 10.1128/JB.01865-14
Mycoplasma pneumoniae, an underutilized model for bacterial cell biology
Abstract
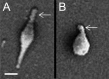
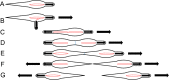
In recent decades, bacterial cell biology has seen great advances, and numerous model systems have been developed to study a wide variety of cellular processes, including cell division, motility, assembly of macromolecular structures, and biogenesis of cell polarity. Considerable attention has been given to these model organisms, which include Escherichia coli, Bacillus subtilis, Caulobacter crescentus, and Myxococcus xanthus. Studies of these processes in the pathogenic bacterium Mycoplasma pneumoniae and its close relatives have also been carried out on a smaller scale, but this work is often overlooked, in part due to this organism's reputation as minimalistic and simple. In this minireview, I discuss recent work on the role of the M. pneumoniae attachment organelle (AO), a structure required for adherence to host cells, in these processes. The AO is constructed from proteins that generally lack homology to those found in other organisms, and this construction occurs in coordination with cell cycle events. The proteins of the M. pneumoniae AO share compositional features with proteins with related roles in model organisms. Once constructed, the AO becomes activated for its role in a form of gliding motility whose underlying mechanism appears to be distinct from that of other gliding bacteria, including Mycoplasma mobile. Together with the FtsZ cytoskeletal protein, motility participates in the cell division process. My intention is to bring this deceptively complex organism into alignment with the better-known model systems.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, Merryman C, Vashee S, Krishnakumar R, Assad-Garcia N, Andrews-Pfannkoch C, Denisova EA, Young L, Qi ZQ, Segall-Shapiro TH, Calvey CH, Parmar PP, Hutchison CA, 3rd, Smith HO, Venter JC. 2010. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329:52–56. 10.1126/science.1190719. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

