The limitations of diazepam as a treatment for nerve agent-induced seizures and neuropathology in rats: comparison with UBP302
- PMID: 25157087
- PMCID: PMC4201270
- DOI: 10.1124/jpet.114.217299
The limitations of diazepam as a treatment for nerve agent-induced seizures and neuropathology in rats: comparison with UBP302
Abstract
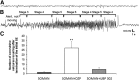
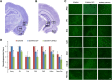
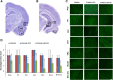
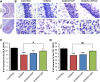
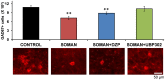
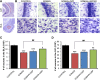
Exposure to nerve agents induces prolonged status epilepticus (SE), causing brain damage or death. Diazepam (DZP) is the current US Food and Drug Administration-approved drug for the cessation of nerve agent-induced SE. Here, we compared the efficacy of DZP with that of UBP302 [(S)-3-(2-carboxybenzyl)willardiine; an antagonist of the kainate receptors that contain the GluK1 subunit] against seizures, neuropathology, and behavioral deficits induced by soman in rats. DZP, administered 1 hour or 2 hours postexposure, terminated the SE, but seizures returned; thus, the total duration of SE within 24 hours after soman exposure was similar to (DZP at 1 hour) or longer than (DZP at 2 hours) that in the soman-exposed rats that did not receive the anticonvulsant. Compared with DZP, UBP302 stopped SE with a slower time course, but dramatically reduced the total duration of SE within 24 hours. Neuropathology and behavior were assessed in the groups that received anticonvulsant treatment 1 hour after exposure. UBP302, but not DZP, reduced neuronal degeneration in a number of brain regions, as well as neuronal loss in the basolateral amygdala and the CA1 hippocampal area, and prevented interneuronal loss in the basolateral amygdala. Anxiety-like behavior was assessed in the open field and by the acoustic startle response 30 days after soman exposure. The results showed that anxiety-like behavior was increased in the DZP-treated group and in the group that did not receive anticonvulsant treatment, but not in the UBP302-treated group. The results argue against the use of DZP for the treatment of nerve agent-induced seizures and brain damage and suggest that targeting GluK1-containing receptors is a more effective approach.
U.S. Government work not protected by U.S. copyright.
Figures




References
-
- Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, Green CE, Swezey R, Yang C, Qashu F, Braga MFM. (2013) Efficacy of the GluK1/AMPA receptor antagonist LY293558 against seizures and neuropathology in a soman-exposure model without pretreatment and its pharmacokinetics after intramuscular administration. J Pharmacol Exp Ther 344:133–140 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

