Regulation of AMPK activation by CD36 links fatty acid uptake to β-oxidation
- PMID: 25157091
- PMCID: PMC4303974
- DOI: 10.2337/db14-0582
Regulation of AMPK activation by CD36 links fatty acid uptake to β-oxidation
Abstract
Increases in muscle energy needs activate AMPK and induce sarcolemmal recruitment of the fatty acid (FA) translocase CD36. The resulting rises in FA uptake and FA oxidation are tightly correlated, suggesting coordinated regulation. We explored the possibility that membrane CD36 signaling might influence AMPK activation. We show, using several cell types, including myocytes, that CD36 expression suppresses AMPK, keeping it quiescent, while it mediates AMPK activation by FA. These dual effects reflect the presence of CD36 in a protein complex with the AMPK kinase LKB1 (liver kinase B1) and the src kinase Fyn. This complex promotes Fyn phosphorylation of LKB1 and its nuclear sequestration, hindering LKB1 activation of AMPK. FA interaction with CD36 dissociates Fyn from the protein complex, allowing LKB1 to remain cytosolic and activate AMPK. Consistent with this, CD36(-/-) mice have constitutively active muscle and heart AMPK and enhanced FA oxidation of endogenous triglyceride stores. The molecular mechanism described, whereby CD36 suppresses AMPK, with FA binding to CD36 releasing this suppression, couples AMPK activation to FA availability and would be important for the maintenance of cellular FA homeostasis. Its dysfunction might contribute to the reported association of CD36 variants with metabolic complications of obesity in humans.
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures

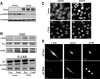

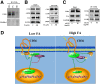
References
-
- Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev 2010;90:367–417 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

