Methylene blue modulates β-secretase, reverses cerebral amyloidosis, and improves cognition in transgenic mice
- PMID: 25157105
- PMCID: PMC4215215
- DOI: 10.1074/jbc.M114.568212
Methylene blue modulates β-secretase, reverses cerebral amyloidosis, and improves cognition in transgenic mice
Abstract
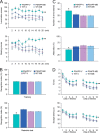
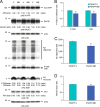
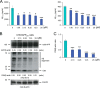
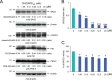
Amyloid precursor protein (APP) proteolysis is required for production of amyloid-β (Aβ) peptides that comprise β-amyloid plaques in the brains of patients with Alzheimer disease (AD). Here, we tested whether the experimental agent methylene blue (MB), used for treatment of methemoglobinemia, might improve AD-like pathology and behavioral deficits. We orally administered MB to the aged transgenic PSAPP mouse model of cerebral amyloidosis and evaluated cognitive function and cerebral amyloid pathology. Beginning at 15 months of age, animals were gavaged with MB (3 mg/kg) or vehicle once daily for 3 months. MB treatment significantly prevented transgene-associated behavioral impairment, including hyperactivity, decreased object recognition, and defective spatial working and reference memory, but it did not alter nontransgenic mouse behavior. Moreover, brain parenchymal and cerebral vascular β-amyloid deposits as well as levels of various Aβ species, including oligomers, were mitigated in MB-treated PSAPP mice. These effects occurred with inhibition of amyloidogenic APP proteolysis. Specifically, β-carboxyl-terminal APP fragment and β-site APP cleaving enzyme 1 protein expression and activity were attenuated. Additionally, treatment of Chinese hamster ovary cells overexpressing human wild-type APP with MB significantly decreased Aβ production and amyloidogenic APP proteolysis. These results underscore the potential for oral MB treatment against AD-related cerebral amyloidosis by modulating the amyloidogenic pathway.
Keywords: Alzheimer Disease; Amyloid; Amyloid Precursor Protein (APP); Animal Model; Anti-amyloidogenic; Methylene Blue; Secretase; Transgenic Mouse; β-Secretase.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Tannic acid is a natural β-secretase inhibitor that prevents cognitive impairment and mitigates Alzheimer-like pathology in transgenic mice.J Biol Chem. 2012 Feb 24;287(9):6912-27. doi: 10.1074/jbc.M111.294025. Epub 2012 Jan 4. J Biol Chem. 2012. PMID: 22219198 Free PMC article.
-
Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice.PLoS One. 2013;8(2):e55774. doi: 10.1371/journal.pone.0055774. Epub 2013 Feb 8. PLoS One. 2013. PMID: 23409038 Free PMC article.
-
Combination therapy with octyl gallate and ferulic acid improves cognition and neurodegeneration in a transgenic mouse model of Alzheimer's disease.J Biol Chem. 2017 Jul 7;292(27):11310-11325. doi: 10.1074/jbc.M116.762658. Epub 2017 May 16. J Biol Chem. 2017. PMID: 28512130 Free PMC article.
-
Evaluation and targeting of amyloid precursor protein (APP)/amyloid beta (Aβ) axis in amyloidogenic and non-amyloidogenic pathways: A time outside the tunnel.Ageing Res Rev. 2023 Dec;92:102119. doi: 10.1016/j.arr.2023.102119. Epub 2023 Nov 4. Ageing Res Rev. 2023. PMID: 37931848 Review.
-
The Role of Proteolysis in Amyloidosis.Int J Mol Sci. 2022 Dec 31;24(1):699. doi: 10.3390/ijms24010699. Int J Mol Sci. 2022. PMID: 36614141 Free PMC article. Review.
Cited by
-
Methylene Blue Improves Brain Mitochondrial ABAD Functions and Decreases Aβ in a Neuroinflammatory Alzheimer's Disease Mouse Model.Mol Neurobiol. 2016 Mar;53(2):1220-1228. doi: 10.1007/s12035-014-9088-8. Epub 2015 Jan 20. Mol Neurobiol. 2016. PMID: 25601181
-
Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice.J Biol Chem. 2020 Nov 27;295(48):16251-16266. doi: 10.1074/jbc.RA119.012330. Epub 2020 Sep 10. J Biol Chem. 2020. PMID: 32913125 Free PMC article.
-
Prion therapeutics: Lessons from the past.Prion. 2022 Dec;16(1):265-294. doi: 10.1080/19336896.2022.2153551. Prion. 2022. PMID: 36515657 Free PMC article. Review.
-
Neuroinflammatory Cytokines Induce Amyloid Beta Neurotoxicity through Modulating Amyloid Precursor Protein Levels/Metabolism.Biomed Res Int. 2018 Oct 25;2018:3087475. doi: 10.1155/2018/3087475. eCollection 2018. Biomed Res Int. 2018. PMID: 30498753 Free PMC article. Review.
-
Pharmacological Agents Targeting the Cellular Prion Protein.Pathogens. 2018 Mar 7;7(1):27. doi: 10.3390/pathogens7010027. Pathogens. 2018. PMID: 29518975 Free PMC article. Review.
References
-
- Brookmeyer R., Gray S. (2000) Methods for projecting the incidence and prevalence of chronic diseases in aging populations: application to Alzheimer's disease. Stat. Med. 19, 1481–1493 - PubMed
-
- Selkoe D. J. (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 81, 741–766 - PubMed
-
- Alzheimer's Disease International (2012) Overcoming the Stigma of Dementia, World Alzheimer Report 2012. Alzheimer's Disease International, London
-
- Rozemuller J. M., Eikelenboom P., Stam F. C., Beyreuther K., Masters C. L. (1989) A4 protein in Alzheimer's disease: primary and secondary cellular events in extracellular amyloid deposition. J. Neuropathol. Exp. Neurol. 48, 674–691 - PubMed
-
- Hardy J., Allsop D. (1991) Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol. Sci. 12, 383–388 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

