Efficacy of Resistance Training as an Aid to Smoking Cessation: Rationale and Design of the Strength To Quit Study
- PMID: 25157265
- PMCID: PMC4141705
- DOI: 10.1016/j.mhpa.2014.05.004
Efficacy of Resistance Training as an Aid to Smoking Cessation: Rationale and Design of the Strength To Quit Study
Abstract
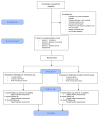
Despite recent declines in the rates of cigarette smoking, smoking remains prevalent among individuals with lower income, less education, and those with mental illness or HIV. Exercise is promoted as an aid to smoking cessation; however, the evidence for this recommendation is equivocal. To date, the majority of studies have only examined aerobic exercise; there is a poor understanding of the mechanisms of action; and there is an under-representation of male smokers. The goal of this trial is to produce new data that will help to address each of these gaps. A total of 206 male and female smokers will receive a brief smoking cessation education session prior to being randomized into a 12-week Resistance Training (RT) or Wellness Contact Control group. Both groups will have the option of using nicotine replacement therapy (NRT), and both will meet on-site twice per week during the 12-week program (24 total sessions). Follow-up assessments will occur at the end of the 12-weeks (3-month), and at a 6-month and 12-month (post-randomization) visit. Participants will not receive any additional smoking cessation treatment during follow-up; however, the RT group will receive a 9-month membership to a fitness center to encourage continued resistance training as a way to maintain cessation, and attendance will be tracked. The primary outcome is salivary-cotinine-verified 7-Day Point Prevalence Abstinence (PPA) at the 3-month assessment, and at the 6 and 12-month follow-ups. Secondary outcomes include effects of resistance training on nicotine withdrawal symptoms, indicators of mental health, and markers of disease risk.
Figures
References
-
- American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 9. Philadelphia, PA: Lippincott Williams & Wilkins; 2014.
-
- Arent SM, Landers DM, Matt KS, Etnier JL. Dose-response and mechanistic issues in the resistance training and affect relationship. Journal of Sport & Exercise Psychology. 2005;27:92–110.
-
- Barry VW, Baruth M, Beets MW, Durstine JL, Liu J, Blair SN. Fitness vs. fatness on all-cause mortality: a meta-analysis. Progress in Cardiovascular Diseases. 2014;56:382–390. Review. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources