Hyaluronan and RHAMM in wound repair and the "cancerization" of stromal tissues
- PMID: 25157350
- PMCID: PMC4137499
- DOI: 10.1155/2014/103923
Hyaluronan and RHAMM in wound repair and the "cancerization" of stromal tissues
Abstract
Tumors and wounds share many similarities including loss of tissue architecture, cell polarity and cell differentiation, aberrant extracellular matrix (ECM) remodeling (Ballard et al., 2006) increased inflammation, angiogenesis, and elevated cell migration and proliferation. Whereas these changes are transient in repairing wounds, tumors do not regain tissue architecture but rather their continued progression is fueled in part by loss of normal tissue structure. As a result tumors are often described as wounds that do not heal. The ECM component hyaluronan (HA) and its receptor RHAMM have both been implicated in wound repair and tumor progression. This review highlights the similarities and differences in their roles during these processes and proposes that RHAMM-regulated wound repair functions may contribute to "cancerization" of the tumor microenvironment.
Figures


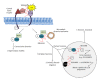
References
-
- Ballard PL, Gonzales LW, Godinez RI, et al. Surfactant composition and function in a primate model of infant chronic lung disease: Effects of inhaled nitric oxide. Pediatric Research. 2006;59(1):157–162. - PubMed
-
- Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nature Reviews Molecular Cell Biology. 2008;9(8):628–638. - PubMed
-
- Veiseh M, Turley EA. Hyaluronan metabolism in remodeling extracellular matrix: probes for imaging and therapy of breast cancer. Integrative Biology. 2011;3(4):304–315. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

