Decrease of U(VI) immobilization capability of the facultative anaerobic strain Paenibacillus sp. JG-TB8 under anoxic conditions due to strongly reduced phosphatase activity
- PMID: 25157416
- PMCID: PMC4144796
- DOI: 10.1371/journal.pone.0102447
Decrease of U(VI) immobilization capability of the facultative anaerobic strain Paenibacillus sp. JG-TB8 under anoxic conditions due to strongly reduced phosphatase activity
Abstract
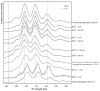
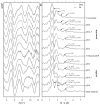
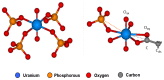
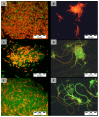
Interactions of a facultative anaerobic bacterial isolate named Paenibacillus sp. JG-TB8 with U(VI) were studied under oxic and anoxic conditions in order to assess the influence of the oxygen-dependent cell metabolism on microbial uranium mobilization and immobilization. We demonstrated that aerobically and anaerobically grown cells of Paenibacillus sp. JG-TB8 accumulate uranium from aqueous solutions under acidic conditions (pH 2 to 6), under oxic and anoxic conditions. A combination of spectroscopic and microscopic methods revealed that the speciation of U(VI) associated with the cells of the strain depend on the pH as well as on the aeration conditions. At pH 2 and pH 3, uranium was exclusively bound by organic phosphate groups provided by cellular components, independently on the aeration conditions. At higher pH values, a part (pH 4.5) or the total amount (pH 6) of the dissolved uranium was precipitated under oxic conditions in a meta-autunite-like uranyl phosphate mineral phase without supplying an additional organic phosphate substrate. In contrast to that, under anoxic conditions no mineral formation was observed at pH 4.5 and pH 6, which was clearly assigned to decreased orthophosphate release by the cells. This in turn was caused by a suppression of the indigenous phosphatase activity of the strain. The results demonstrate that changes in the metabolism of facultative anaerobic microorganisms caused by the presence or absence of oxygen can decisively influence U(VI) biomineralization.
Conflict of interest statement
Figures


Similar articles
-
Bio-precipitation of uranium by two bacterial isolates recovered from extreme environments as estimated by potentiometric titration, TEM and X-ray absorption spectroscopic analyses.J Hazard Mater. 2011 Dec 15;197:1-10. doi: 10.1016/j.jhazmat.2011.09.049. Epub 2011 Oct 2. J Hazard Mater. 2011. PMID: 22019055
-
Aerobic uranium immobilization by Rhodanobacter A2-61 through formation of intracellular uranium-phosphate complexes.Metallomics. 2013 Apr;5(4):390-7. doi: 10.1039/c3mt00052d. Metallomics. 2013. PMID: 23487302
-
Interaction of Uranium with Bacterial Cell Surfaces: Inferences from Phosphatase-Mediated Uranium Precipitation.Appl Environ Microbiol. 2016 Jul 29;82(16):4965-74. doi: 10.1128/AEM.00728-16. Print 2016 Aug 15. Appl Environ Microbiol. 2016. PMID: 27287317 Free PMC article.
-
Engineering and kinetic aspects of bacterial uranium reduction for the remediation of uranium contaminated environments.J Hazard Mater. 2019 Jun 5;371:198-212. doi: 10.1016/j.jhazmat.2019.02.074. Epub 2019 Feb 21. J Hazard Mater. 2019. PMID: 30851673 Review.
-
Uranium speciation and bioavailability in aquatic systems: an overview.ScientificWorldJournal. 2002 Mar 15;2:707-29. doi: 10.1100/tsw.2002.130. ScientificWorldJournal. 2002. PMID: 12805996 Free PMC article. Review.
Cited by
-
Transcriptome Response of the Tropical Marine Yeast Yarrowia lipolytica on Exposure to Uranium.Curr Microbiol. 2021 May;78(5):2033-2043. doi: 10.1007/s00284-021-02459-z. Epub 2021 Mar 27. Curr Microbiol. 2021. PMID: 33772621
-
Radioactivity as a driver of bacterial community composition in naturally radioactive mineral springs in the French Massif Central.Front Microbiol. 2024 Jul 23;15:1423342. doi: 10.3389/fmicb.2024.1423342. eCollection 2024. Front Microbiol. 2024. PMID: 39109212 Free PMC article.
-
Impact of microbial processes on the safety of deep geological repositories for radioactive waste.Front Microbiol. 2023 Mar 16;14:1134078. doi: 10.3389/fmicb.2023.1134078. eCollection 2023. Front Microbiol. 2023. PMID: 37007474 Free PMC article. Review.
-
Molecular Mechanisms Underlying Bacterial Uranium Resistance.Front Microbiol. 2022 Mar 10;13:822197. doi: 10.3389/fmicb.2022.822197. eCollection 2022. Front Microbiol. 2022. PMID: 35359714 Free PMC article. Review.
-
Transposon Mutagenesis Paired with Deep Sequencing of Caulobacter crescentus under Uranium Stress Reveals Genes Essential for Detoxification and Stress Tolerance.J Bacteriol. 2015 Oct;197(19):3160-72. doi: 10.1128/JB.00382-15. Epub 2015 Jul 20. J Bacteriol. 2015. PMID: 26195598 Free PMC article.
References
-
- Ahearne JF (1997) Radioactive waste: The size of the problem. Phys Today 50: 24–29.
-
- Murphy WM, Shock EL (1999) Environmental aqueous geochemistry of actinides. Rev Mineral 38: 221–253.
-
- Baik MH, Hyun SP, Cho WJ, Hahn PS (2004) Contribution of minerals to the sorption of U(VI) on granite. Radiochim Acta 92: 663–669.
-
- Barnett MO, Jardine PM, Brooks SC, Selim HM (2000) Adsorption and transport of uranium(VI) in subsurface media. Soil Sci Soc Am J 64: 908–917.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

