Methotrexate for maintenance of remission in Crohn's disease
- PMID: 25157445
- PMCID: PMC8202560
- DOI: 10.1002/14651858.CD006884.pub3
Methotrexate for maintenance of remission in Crohn's disease
Abstract
Background: Safe and effective long-term treatments that reduce the need for corticosteroids are needed for Crohn's disease. Although purine antimetabolites are moderately effective for maintenance of remission patients often relapse despite treatment with these agents. Methotrexate may provide a safe and effective alternative to more expensive maintenance treatment with TNF-α antagonists. This review is an update of a previously published Cochrane review.
Objectives: To conduct a systematic review of randomized trials examining the efficacy and safety of methotrexate for maintenance of remission in Crohn's disease.
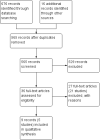
Search methods: The Cochrane Central Register of Controlled Trials (CENTRAL), PUBMED, EMBASE, and the Cochrane IBD/FBD Group Specialized Trials Register were searched from inception to June 9, 2014. Study references and review papers were also searched for additional trials.
Selection criteria: Randomised controlled trials (RCTs) that compared methotrexate to placebo or any other active intervention for maintenance of remission in Crohn's disease were eligible for inclusion.
Data collection and analysis: Two authors independently reviewed studies for eligibility, extracted data and assessed study quality using the Cochrane risk of bias tool. The primary outcome measure was the proportion of patients maintaining clinical remission as defined by the studies and expressed as a percentage of the total number of patients randomized (intention-to-treat analysis). We calculated the pooled risk ratio (RR) and corresponding 95% confidence intervals (95% CI) for dichotomous outcomes. The overall quality of the evidence supporting the primary outcome was assessed using the GRADE criteria.
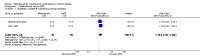
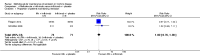
Main results: Five studies (n = 333 patients) were included in the review. Three studies were judged to be at low risk of bias. Two studies were judged to be at high risk of bias due to blinding. Intramuscular methotrexate was superior to placebo for maintenance of remission at 40 weeks follow-up. Sixty-five per cent of patients in the intramuscular methotrexate group maintained remission compared to 39% of placebo patients (RR 1.67, 95% CI 1.05 to 2.67; 76 patients).The number needed to treat to prevent one relapse was four. A GRADE analysis indicated that the overall quality of evidence supporting this outcome was moderate due to sparse data (40 events). There was no statistically significant difference in maintenance of remission at 36 weeks follow-up between oral methotrexate (12.5 mg/week) and placebo. Ninety per cent of patients in the oral methotrexate group maintained remission compared to 67% of placebo patients (RR 1.67, 95% CI 1.05 to 2.67; 22 patients). A GRADE analysis indicated that the overall quality of evidence supporting this outcome was low due to very sparse data (17 events). A pooled analysis of two small studies (n = 50) showed no statistically significant difference in continued remission between oral methotrexate (12.5 mg to 15 mg/week) and 6-mercaptopurine (1 mg/kg/day) for maintenance of remission. Seventy-seven per cent of methotrexate patients maintained remission compared to 57% of 6-mercaptopurine patients (RR 1.36, 95% CI 0.92 to 2.00). A GRADE analysis indicated that the overall quality of evidence supporting this outcome was very low due to high risk of bias in one study (no blinding) and very sparse data (33 events). One small (13 patients) poor quality study found no difference in continued remission between methotrexate and 5-aminosalicylic acid (RR 2.62, 95% CI 0.23 to 29.79). A pooled analysis of two studies (n = 145) including one high quality trial (n = 126) found no statistically significant difference in maintenance of remission at 36 to 48 weeks between combination therapy (methotrexate and infliximab) and infliximab monotherapy. Fifty-four percent of patients in the combination therapy group maintained remission compared to 53% of monotherapy patients (RR 1.02, 95% CI 0.76 to 1.38, P = 0.95). A GRADE analysis indicated that the overall quality of evidence supporting this outcome was low due to high risk of bias in one study (no blinding) and sparse data (78 events). Adverse events were generally mild in nature and resolved upon discontinuation or with folic acid supplementation. Common adverse events included nausea and vomiting, symptoms of a cold, abdominal pain, headache, joint pain or arthralgia, and fatigue.
Authors' conclusions: Moderate quality evidence indicates that intramuscular methotrexate at a dose of 15 mg/week is superior to placebo for maintenance of remission in Crohn's disease. Intramuscular methotrexate appears to be safe. Low dose oral methotrexate (12.5 to 15 mg/week) does not appear to be effective for maintenance of remission in Crohn's disease. Combination therapy (methotrexate and infliximab) does not appear to be any more effective for maintenance of remission than infliximab monotherapy. The results for efficacy outcomes between methotrexate and 6-mercaptopurine and methotrexate and 5-aminosalicylic acid were uncertain. Large-scale studies of methotrexate given orally at higher doses for maintenance of remission in Crohn's disease may provide stronger evidence for the use of methotrexate in this manner.
Conflict of interest statement
Nilesh Chande has received fees for consultancy from Abbott/AbbVie and Ferring, fees for lectures from Abbott and Janssen, travel expenses from Merck and has stock/stock options in Pfizer, Glaxo Smith Kline, Proctor and Gamble and Johnson and Johnson. All of these financial activities are outside the submitted work.
John WD McDonald was a coauthor of one of the original publications reviewed in the preparation of this Cochrane review.
The other authors have no known declarations of interest.
Figures


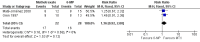
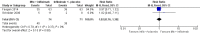


Update of
-
Methotrexate for maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2009 Oct 7;(4):CD006884. doi: 10.1002/14651858.CD006884.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2014 Aug 26;(8):CD006884. doi: 10.1002/14651858.CD006884.pub3. PMID: 19821390 Updated.
References
References to studies included in this review
Feagan 2000 {published data only}
-
- Feagan BG, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. New England Journal of Medicine 2000;342(22):1627‐32. [PUBMED: 2000197470] - PubMed
-
- Feagan BG, Fedorak RN, Irvine EJ, Wild GE, Sutherland LR, Steinhart AH, et al. A comparison of methotrexate with placebo for the maintenance for the maintenance of remission in Crohn's disease. Gastroenterology 2000;118(4 Suppl 2):A190. - PubMed
Feagan 2014 {published data only}
-
- Feagan B, McDonald JW, Panaccione R, Enns RA, Bernstein CN, Ponich TP, et al. A randomized trial of methotrexate in combination with infliximab for the treatment of Crohn’s disease. Gastroenterology 2008;135(1):294‐5.
-
- Feagan BG, McDonald JWD, Panaccione R, Enns R, Bernstein CN, Ponich T, et al. A randomized trial of methotrexate in combination with infliximab for the treatment of Crohn's disease. United European Gastroenterology Week. Vienna, Austria, 2008:Abstract OP301.
-
- Feagan BG, McDonald JWD, Panaccione R, Enns RA, Bernstein CN, Ponich TP, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn's disease. Gastroenterology 2014;146(3):681‐8. [PUBMED: 24269926] - PubMed
Maté‐Jiménez 2000 {published data only}
-
- Hermida C, Cantero J, Moreno‐Otero R, Maté‐Jiménez J. Methotrexate and 6‐mercaptopurine in steroid‐dependent inflammatory bowel disease patients: a randomized controlled clinical trial. 1999 Gut;45(Suppl V):A132.
-
- Maté‐Jiménez J, Hermida C, Cantero‐Perona J, Moreno‐Otero R. 6‐Mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid‐dependent inflammatory bowel disease. European Journal of Gastroenterology and Hepatology 2000;12(11):1227‐33. [PUBMED: 11111780] - PubMed
Oren 1997 {published data only}
-
- Oren R, Moshkowitz M, Odes S, Becker S, Keter D, Pomeranz I, et al. Methotrexate in chronic active Crohn's disease: a double‐blind, randomized, Israeli multicenter trial. American Journal of Gastroenterology 1997;92(12):2203‐9. [PUBMED: 1997381066] - PubMed
Schröder 2006 {published data only}
-
- Schröder O, Blumenstein I, Stein J. Combining infliximab with methotrexate for the induction and maintenance of remission in refractory Crohn's disease: a controlled pilot study. European Journal of Gastroenterology and Hepatology 2006;18(1):11‐6. [PUBMED: 16357613] - PubMed
References to studies excluded from this review
Ardizzone 2003 {published data only}
-
- Ardizzone S, Bollani S, Manzionna G, Imbesi V, Colombo E, Bianchi Porro G. Comparison between methotrexate and azathioprine in the treatment of chronic active Crohn's disease: a randomised, investigator‐blind study. Digestive and Liver Disease 2003;35(9):619‐27. [PUBMED: 14563183] - PubMed
-
- Ardizzone S, Bollani S, Manzionna G, Molteni P, Bareggi E, Bianchi Porro G. Controlled trial comparing intravenous methotrexate and oral azathioprine for chronic active Crohn's disease: preliminary report. Gastroenterology 1999;116(4 (Part 2)):A662‐3.
-
- Bianchi Porro G, Ardizzone S, Bollani S, Duca A, Manzionna G, Molteni P. Controlled trial comparing intravenous methotrexate and oral azathioprine for chronic active Crohn's disease: preliminary report. Gut 1982;23(10):295.
Arora 1999 {published data only}
-
- Arora S, Katkov W, Cooley J, Kemp JA, Johnston DE, Schapiro RH, et al. Methotrexate in Crohn's disease: results of a randomized, double‐blind, placebo‐controlled trial. Hepatogastroenterology 1999;46(27):1724‐9. [PUBMED: 10430331] - PubMed
-
- Arora S, Katkov WN, Cooley J, Kemp A, Schapiro RH, Kelsey PB, et al. A double blind, randomized, placebo‐controlled trial of methotrexate in Crohn's disease. Gastroenterology 1992;102(4 Part 2):A591. - PubMed
Clark 2005 {published data only}
-
- Clark LL, Lightbody E, Morgan A, Gaya D, Winter JW, Gillespie RJ. The efficacy of long term intramuscular methotrexate in difficult to treat Crohn's disease. Gastroenterology 2005;136(5 Suppl 1):A659.
Domènech 2008 {published data only}
-
- Domènech E, Mañosa M, Navarro M, Masnou H, Garcia‐Planella E, Zabana Y, et al. Long‐term methotrexate for Crohn's disease: safety and efficacy in clinical practice. Journal of Clinical Gastroenterology 2008;42(4):395‐9. [PUBMED: 18277899] - PubMed
Egan 1999a {published data only}
-
- Egan L, Sandborn W, Tremaine W, Leighton J, Mays D, Pike M, et al. A randomized, single‐blind, pharmacokinetic and dose response study of subcutaneous methotrexate, 15 and 25 mg/week, for refractory ulcerative colitis and Crohn's disease. Gastroenterology 1998;114(4 Pt2):A‐227.
-
- Egan LJ, Sandborn WJ, Tremaine WJ, Leighton JA, Mays DC, Pike MG, et al. A randomized dose‐response and pharmacokinetic study of methotrexate for refractory inflammatory Crohn's disease and ulcerative colitis. Alimentary Pharmacology and Therapeutics 1999;13(12):1597‐604. [PUBMED: 10594394] - PubMed
Feagan 1995 {published data only}
-
- Feagan BG, North American Crohn's Study Group Investigators. A Multicentre trial of methotrexate (MTX) for chronically active Crohn's disease (CD). Gut 1994;35 Suppl 4:A121.
-
- Feagan BG, Rochon J, Fedorak RN, Irvine EJ, Wild G, Sutherland L, et al. Methotrexate for the treatment of Crohn's disease. New England Journal of Medicine 1995;332(5):292‐7. [PUBMED: 7816064] - PubMed
-
- Investigators, T.N.A.C.s.S.G. A multicentre trial of methotrexate (MTX) treatment for chronically active Crohn's disease. Gastroenterology 1994;106(4 Part 2):A745.
Fishman 2001 {published data only}
-
- Fishman M. Methotrexate and maintenance of remission in Crohn's disease. Canadian Journal of Gastroenterology 2001;15(7):428. [PUBMED: 11493943] - PubMed
Hayee 2005 {published data only}
-
- Hayee BH, Harris AW. Methotrexate for Crohn's disease: experience in a district general hospital. European Journal of Gastroenterology and Hepatology 2005;17(9):893‐8. [PUBMED: 16093864] - PubMed
Houben 1994 {published data only}
-
- Houben MH, Wijk HJ, Driessen WM, Spreeuwel JP. Methotrexate as possible treatment in refractory chronic inflammatory intestinal disease. Nederlands Tijdschrift voor Geneeskunde 1994;138(51):2552‐6. [PUBMED: 7830804] - PubMed
Kurnik 2003 {published data only}
-
- Kurnik D, Loebstein R, Fishbein E, Almog S, Halkin H, Bar‐Meir S, et al. Bioavailability of oral vs. subcutaneous low‐dose methotrexate in patients with Crohn's disease. Alimentary Pharmacology and Therapeutics 2003;18(1):57‐63. [PUBMED: 12848626] - PubMed
Lahaire 2011 {published data only}
-
- Laharie D, Reffet A, Belleannée G, Chabrun E, Subtil C, Razaire S. Mucosal healing with methotrexate in Crohn's disease: a prospective comparative study with azathioprine and infliximab. Alimentary Pharmacology and Therapeutics 2011;33(6):714‐21. [PUBMED: 21235604] - PubMed
Lampen‐Smith 2011 {published data only}
-
- Lampen‐Smith A, Khan I, Claydon A. Methotrexate in patients with Crohn's disease: a regional experience. Journal of Gastroenterology and Hepatology 2011;26:61‐2. [PUBMED: 70627238]
Lémann 2000 {published data only}
-
- Lémann M, Zenjari T, Bouhnik Y, Cosnes J, Mesnard B, Rambaud JC, et al. Methotrexate in Crohn's disease: long‐term efficacy and toxicity. American Journal of Gastroenterology 2000;95(7):1730‐4. [PUBMED: 10925976] - PubMed
Martreau 2000 {published data only}
-
- Martreau P. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. North American Crohn's Study Group Investigators [Demonstration de l'efficacite du methotrexate par voie parenterale pour maintenir en remission la maladie de Crohn]. Gastroenterologie Clinique et Biologique 2000;24(12):1243‐4. [PUBMED: 11277090] - PubMed
Roseau 2000 {published data only}
-
- Roseau E. Crohn's disease: prevention of relapse with methotrexate or growth hormone [Maladie de Crohn: Prevention des rechutes par le methotrexate ou l'hormone de croissance]. Presse Medicale 2000;29(30):1652‐3. [PUBMED: 11089504] - PubMed
Roznowski 2001 {published data only}
-
- Roznowski AB, Dignass A. Sustaining remission in Crohn disease with methotrexate‐‐a placebo controlled study [Remissionserhaltung bei Morbus Crohn mit Methotrexat‐‐eine plazebokontrollierte Untersuchung]. Zeitschrift fur Gastroenterologie 2001;39(3):265‐7. [PUBMED: 11324144] - PubMed
Schröder 1996 {published data only}
-
- Schröder O, Stein J. Methotrexate in therapy of chronic inflammatory bowel diseases [Methotrexat (MTX) in der Therapie chronische‐entzundlicher Darmerkrankungen]. Zeitschrift fur Gastroenterologie 1996;34(7):457‐8. [PUBMED: 8928541] - PubMed
Suares 2012 {published data only}
-
- Suares NC, Hamlin PJ, Greer DP, Warren L, Clark T, Ford AC. Efficacy and tolerability of methotrexate therapy for refractory Crohn's disease: a large single‐centre experience. Alimentary Pharmacology and Therapeutics 2012;35(2):284‐91. [PUBMED: 22112005] - PubMed
Sun 2005 {published data only}
-
- Sun JH, Das KM. Low‐dose oral methotrexate for maintaining Crohn's disease remission: where we stand. Journal of Clinical Gastroenterology 2005;39(9):751‐6. [PUBMED: 16145336] - PubMed
Wilson 2013 {published data only}
-
- Wilson A, Patel V, Chande N, Ponich T, Urquhart B, Asher L, et al. Pharmacokinetic profiles for oral and subcutaneous methotrexate in patients with Crohn's disease. Alimentary Pharmacology and Therapeutics 2013;37(3):340‐5. [PUBMED: 23190184] - PubMed
Yang 2001 {published data only}
-
- Yang YX, Lichtenstein GR. Methotrexate for the maintenance of remission in Crohn's disease. Gastroenterology 2001;120(6):1553‐5. [PUBMED: 11313329] - PubMed
Additional references
Arthur 2002
-
- Arthur V, Jubb R, Homer D. A study of parenteral use of methotrexate in rheumatic conditions. Journal of Clinical Nursing 2002;11(2):256‐63. [PUBMED: 11903725] - PubMed
Baert 2003
-
- Baert F, Noman M, Vermeire S, Assche G, D' Haens G, Carbonez A, et al. Influence of immunogenicity on the long‐term efficacy of infliximab in Crohn's disease. New England Journal of Medicine 2003;348(7):601‐8. [PUBMED: 12584368] - PubMed
Balis 1988
-
- Balis FM, Mirro J Jr, Reaman GH, Evans WE, McCully C, Doherty KM, et al. Pharmacokinetics of subcutaneous methotrexate. Journal of Clinical Oncology 1988;6(12):1882‐6. [PUBMED: 3199171] - PubMed
Chebli 2007
-
- Chebli JM, Gaburri PD, Souza AF, Pinto AL, Chebli LA, Felga GE, et al. Long‐term results with azathioprine therapy in patients with corticosteroid‐dependent Crohn's disease: open‐label prospective study. Journal of Gastroenterology and Hepatology 2007;22(2):268‐74. [PUBMED: 17295882] - PubMed
Egan 1999b
-
- Egan LJ, Sandborn WJ, Mays DC, Tremaine WJ, Fauq AH, Lipsky JJ. Systemic and intestinal pharmacokinetics of methotrexate in patients with inflammatory bowel disease. Clinical Pharmacology and Therapeutics 1999;65(1):29‐39. [PUBMED: 9951428] - PubMed
Guyatt 2008
Hanauer 2002
-
- Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002;359(9317):1541‐9. [PUBMED: 12047962] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lichtenstein 2006
-
- Lichtenstein GR, Abreu MT, Cohen R, Tremaine W, American Gastroenterological Association. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006;130(3):935‐9. [PUBMED: 16530531] - PubMed
McDonald 2014
Prefontaine 2009
Rampton 2001
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

