Effect of chlorogenic acid on melanogenesis of B16 melanoma cells
- PMID: 25157464
- PMCID: PMC6271456
- DOI: 10.3390/molecules190912940
Effect of chlorogenic acid on melanogenesis of B16 melanoma cells
Abstract
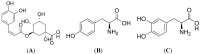
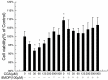
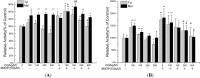
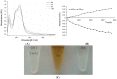
Chlorogenic acid (CGA), the ester formed between caffeic acid and l-quinic acid, is a widespread phenolic compound. It is part of the human diet, found in foods such as coffee, apples, pears, etc. CGA is also was widely used in cosmetics, but the effects of CGA on melanogenesis are unknown. In this study, we analyzed the effects of CGA on cell proliferation, melanin content and tyrosinase of B16 murine melanoma cells. Additionally, the enzymatic reactions of CGA in B16 melanoma cells lytic solution were detected by UV spectrophotometry. Results showed CGA at 30 and 60 μM significantly suppresses cell proliferation. 8-MOP at 100 μM significantly promotes cell proliferation, but CGA can counter this. Incubated for 24 h, CGA (500 μM) improves melanogenesis while suppressing tyrosinase activity in B16 melanoma cells or 8-methoxypsoralen (8-MOP) co-incubated B16 melanoma cells. After 12 h, B16 melanoma cell treatment with CGA leads to an increase in melanin accumulation, however, after 48 h there is a decrease in melanin production which correlates broadly with a decrease in tyrosinase activity. CGA incubated with lytic solution 24 h turned brown at 37 °C. The formation of new products (with a maximum absorption at 295 nm) is associated with reduction of CGA (maximum absorption at 326 nm). Therefore, CGA has its two sidesroles in melanogenesis of B16 melanoma cells. CGA is a likely a substrate of melanin, but the metabolic product(s) of CGA may suppress melanogenesis in B16 melanoma cells by inhibiting tyrosinase activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Diethylstilbestrol enhances melanogenesis via cAMP-PKA-mediating up-regulation of tyrosinase and MITF in mouse B16 melanoma cells.Steroids. 2011 Nov;76(12):1297-304. doi: 10.1016/j.steroids.2011.06.008. Epub 2011 Jun 30. Steroids. 2011. PMID: 21745488
-
Ethyl acetate extract from Panax ginseng C.A. Meyer and its main constituents inhibit α-melanocyte-stimulating hormone-induced melanogenesis by suppressing oxidative stress in B16 mouse melanoma cells.J Ethnopharmacol. 2017 Aug 17;208:149-156. doi: 10.1016/j.jep.2017.07.004. Epub 2017 Jul 8. J Ethnopharmacol. 2017. PMID: 28689798
-
Regulation of melanogenesis induced by 5-methoxypsoralen without ultraviolet light in murine melanoma cells.Pigment Cell Res. 1994 Aug;7(4):245-54. doi: 10.1111/j.1600-0749.1994.tb00059.x. Pigment Cell Res. 1994. PMID: 7855073
-
Inhibition of melanogenesis in murine B16/F10 melanoma cells by Ligusticum sinensis Oliv.Am J Chin Med. 2006;34(3):523-33. doi: 10.1142/S0192415X06004053. Am J Chin Med. 2006. PMID: 16710901
-
[Inhibitory effect of arbutin on melanogenesis--biochemical study using cultured B16 melanoma cells].Nihon Hifuka Gakkai Zasshi. 1991 May;101(6):609-13. Nihon Hifuka Gakkai Zasshi. 1991. PMID: 1920891 Japanese.
Cited by
-
A Systematic Study of Yiqi Qubai Standard Decoction for Treating Vitiligo Based on UPLC-Q-TOF/MS Combined with Chemometrics, Molecular Docking, and Cellular and Zebrafish Assays.Pharmaceuticals (Basel). 2023 Dec 11;16(12):1716. doi: 10.3390/ph16121716. Pharmaceuticals (Basel). 2023. PMID: 38139842 Free PMC article.
-
Investigation of the profile of phenolic compounds in the leaves and stems of Pandiaka heudelotii using gas chromatography coupled with flame ionization detector.Food Sci Nutr. 2016 Nov 23;5(3):646-652. doi: 10.1002/fsn3.443. eCollection 2017 May. Food Sci Nutr. 2016. PMID: 28572953 Free PMC article.
-
Neobavaisoflavone Inhibits Melanogenesis through the Regulation of Akt/GSK-3β and MEK/ERK Pathways in B16F10 Cells and a Reconstructed Human 3D Skin Model.Molecules. 2020 Jun 9;25(11):2683. doi: 10.3390/molecules25112683. Molecules. 2020. PMID: 32527040 Free PMC article.
-
Anti-Melanogenic Properties of Greek Plants. A Novel Depigmenting Agent from Morus alba Wood.Molecules. 2017 Mar 23;22(4):514. doi: 10.3390/molecules22040514. Molecules. 2017. PMID: 28333105 Free PMC article.
-
Fast Screening of Tyrosinase Inhibitors in Coreopsis tinctoria Nutt. by Ligand Fishing Based on Paper-Immobilized Tyrosinase.Molecules. 2024 Aug 25;29(17):4018. doi: 10.3390/molecules29174018. Molecules. 2024. PMID: 39274866 Free PMC article.
References
-
- Scharffetter-Kochanek K., Wlaschek M., Brenneisen P., Schauen M., Blaudschun R., Wenk J. UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biol. Chem. 1997;378:1247–1257. - PubMed
-
- Gaudout D., Megard D., Inisan C., Esteve C., Lejard F. Phloridzin-Rich Phenolic Fraction and Use Thereof as a Cosmetic, Dietary or Nutraceutical Agent. 7427418 B2. U.S. Patent. 2008 Sep 23;
-
- Gaudout D., Megard D., Lejard F. Use of a Dihydrochalcone-Rich Phenolic Fraction in a Cosmetic Treatment. 7285298. U.S. Patent. 2007 Oct 23;
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

