Synthesis of phosphorescent asymmetrically π-extended porphyrins for two-photon applications
- PMID: 25157580
- PMCID: PMC4168792
- DOI: 10.1021/jo501521x
Synthesis of phosphorescent asymmetrically π-extended porphyrins for two-photon applications
Abstract
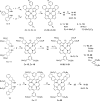
Significant effort has been directed in recent years toward porphyrins with enhanced two-photon absorption (2PA). However, the properties of their triplet states, which are central to many applications, have rarely been examined in parallel. Here we report the synthesis of asymmetrically π-extended platinum(II) and palladium(II) porphyrins, whose 2PA into single-photon-absorbing states is enhanced as a result of the broken center-of-inversion symmetry and whose triplet states can be monitored by room-temperature phosphorescence. 5,15-Diaryl-syn-dibenzoporphyrins (DBPs) and syn-dinaphthoporphyrins (DNPs) were synthesized by [2 + 2] condensation of the corresponding dipyrromethanes and subsequent oxidative aromatization. Butoxycarbonyl groups on the meso-aryl rings render these porphyrins well-soluble in a range of organic solvents, while 5,15-meso-aryl substitution causes minimal nonplanar distortion of the macrocycle, ensuring high triplet emissivity. A syn-DBP bearing four alkoxycarbonyl groups in the benzo rings and possessing a large static dipole moment was also synthesized. Photophysical properties (2PA brightness and phosphorescence quantum yields and lifetimes) of the new porphyrins were measured, and their ground-state structures were determined by DFT calculations and/or X-ray analysis. The developed synthetic methods should facilitate the construction of π-extended porphyrins for applications requiring high two-photon triplet action cross sections.
Figures







References
-
- Lash T. D. In The Porphyrin Handbook; Kadish K. M., Smith K. M., Guilard R., Eds.; Academic Press: New York, 2000; p 125.
-
- Cheprakov A. V. In Handbook of Porphyrin Science; Kadish K. M., Smith K. M., Guilard R., Eds.; World Scientific: Singapore, 2011; Vol. 13, p 1.
-
- Galanin N. E.; Shaposhnikov G. P.; Koifman O. I. Russ. Chem. Rev. 2013, 82, 412.
-
- Carvalho C. M. B.; Brocksom T. J.; de Oliveira K. T. Chem. Soc. Rev. 2013, 42, 3302. - PubMed
-
- Chen P. L.; Tomov I. V.; Dvornikov A. S.; Nakashima M.; Roach J. F.; Alabran D. M.; Rentzepis P. M. J. Phys. Chem. 1996, 100, 17507.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

