Human erythrocyte band 3 functions as a receptor for the sialic acid-independent invasion of Plasmodium falciparum. Role of the RhopH3-MSP1 complex
- PMID: 25157665
- PMCID: PMC4198471
- DOI: 10.1016/j.bbamcr.2014.08.008
Human erythrocyte band 3 functions as a receptor for the sialic acid-independent invasion of Plasmodium falciparum. Role of the RhopH3-MSP1 complex
Abstract
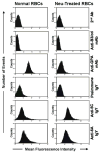
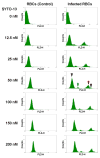
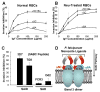
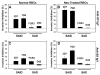
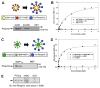
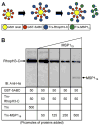
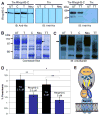
Plasmodium falciparum takes advantage of two broadly defined alternate invasion pathways when infecting human erythrocytes: one that depends on and the other that is independent of host sialic acid residues on the erythrocyte surface. Within the sialic acid-dependent (SAD) and sialic acid-independent (SAID) invasion pathways, several alternate host receptors are used by P. falciparum based on its particular invasion phenotype. Earlier, we reported that two putative extracellular regions of human erythrocyte band 3 termed 5C and 6A function as host invasion receptor segments binding parasite proteins MSP1 and MSP9 via a SAID mechanism. In this study, we developed two mono-specific anti-peptide chicken IgY antibodies to demonstrate that the 5C and 6A regions of band 3 are exposed on the surface of human erythrocytes. These antibodies inhibited erythrocyte invasion by the P. falciparum 3D7 and 7G8 strains (SAID invasion phenotype), and the blocking effect was enhanced in sialic acid-depleted erythrocytes. In contrast, the IgY antibodies had only a marginal inhibitory effect on FCR3 and Dd2 strains (SAD invasion phenotype). A direct biochemical interaction between erythrocyte band 3 epitopes and parasite RhopH3, identified by the yeast two-hybrid screen, was established. RhopH3 formed a complex with MSP119 and the 5ABC region of band 3, and a recombinant segment of RhopH3 inhibited parasite invasion in human erythrocytes. Together, these findings provide evidence that erythrocyte band 3 functions as a major host invasion receptor in the SAID invasion pathway by assembling a multi-protein complex composed of parasite ligands RhopH3 and MSP1.
Keywords: Band 3; Chicken antibody; Erythrocyte; MSP1; Plasmodium falciparum; RhopH3.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures



References
-
- Oh SS, Chishti AH. Host receptors in malaria merozoite invasion. Curr Top Microbiol Immunol. 2005;295:203–232. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

