Gabapentin attenuates hyperexcitability in the freeze-lesion model of developmental cortical malformation
- PMID: 25158291
- PMCID: PMC4179994
- DOI: 10.1016/j.nbd.2014.08.022
Gabapentin attenuates hyperexcitability in the freeze-lesion model of developmental cortical malformation
Abstract
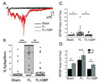
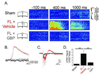
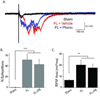
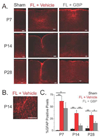
Developmental cortical malformations are associated with a high incidence of drug-resistant epilepsy. The underlying epileptogenic mechanisms, however, are poorly understood. In rodents, cortical malformations can be modeled using neonatal freeze-lesion (FL), which has been shown to cause in vitro cortical hyperexcitability. Here, we investigated the therapeutic potential of gabapentin, a clinically used anticonvulsant and analgesic, in preventing FL-induced in vitro and in vivo hyperexcitability. Gabapentin has been shown to disrupt the interaction of thrombospondin (TSP) with α2δ-1, an auxiliary calcium channel subunit. TSP/α2δ-1 signaling has been shown to drive the formation of excitatory synapses during cortical development and following injury. Gabapentin has been reported to have neuroprotective and anti-epileptogenic effects in other models associated with increased TSP expression and reactive astrocytosis. We found that both TSP and α2δ-1 were transiently upregulated following neonatal FL. We therefore designed a one-week GBP treatment paradigm to block TSP/α2δ-1 signaling during the period of their upregulation. GBP treatment prevented epileptiform activity following FL, as assessed by both glutamate biosensor imaging and field potential recording. GBP also attenuated FL-induced increases in mEPSC frequency at both P7 and 28. Additionally, GBP treated animals had decreased in vivo kainic acid (KA)-induced seizure activity. Taken together these results suggest gabapentin treatment immediately after FL can prevent the formation of a hyperexcitable network and may have therapeutic potential to minimize epileptogenic processes associated with developmental cortical malformations.
Keywords: Cortex; Developmental cortical malformation; Epilepsy; Freeze lesion; Gabapentin; Glutamate; Thrombospondin.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



References
-
- Bordey A, Lyons SA, Hablitz JJ, Sontheimer H. Electrophysiological characteristics of reactive astrocytes in experimental cortical dysplasia. J Neurophys. 2001;85:1719–1731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

