Development of multisensory integration from the perspective of the individual neuron
- PMID: 25158358
- PMCID: PMC4215474
- DOI: 10.1038/nrn3742
Development of multisensory integration from the perspective of the individual neuron
Abstract
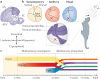
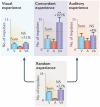
The ability to use cues from multiple senses in concert is a fundamental aspect of brain function. It maximizes the brain’s use of the information available to it at any given moment and enhances the physiological salience of external events. Because each sense conveys a unique perspective of the external world, synthesizing information across senses affords computational benefits that cannot otherwise be achieved. Multisensory integration not only has substantial survival value but can also create unique experiences that emerge when signals from different sensory channels are bound together. However, neurons in a newborn’s brain are not capable of multisensory integration, and studies in the midbrain have shown that the development of this process is not predetermined. Rather, its emergence and maturation critically depend on cross-modal experiences that alter the underlying neural circuit in such a way that optimizes multisensory integrative capabilities for the environment in which the animal will function.
Figures





References
-
- Stein BE, Meredith MA. The Merging of the Senses. MIT Press; 1993.
-
- Alais D, Burr D. The ventriloquist effect results from near-optimal bimodal integration. Curr. Biol. 2004;14:257–262. - PubMed
-
- Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature. 2002;415:429–433. - PubMed
-
- Shams L, Ma WJ, Beierholm U. Sound-induced flash illusion as an optimal percept. Neuroreport. 2005;16:1923–1927. - PubMed
-
- Lewkowicz DJ, Lickliter R, editors. The Development of Intersensory Perception: Comparative Perspectives. Lawrence Erlbaum Associates; 1994.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

