Actin-microtubule coordination at growing microtubule ends
- PMID: 25159196
- PMCID: PMC4365169
- DOI: 10.1038/ncomms5778
Actin-microtubule coordination at growing microtubule ends
Abstract
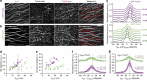
To power dynamic processes in cells, the actin and microtubule cytoskeletons organize into complex structures. Although it is known that cytoskeletal coordination is vital for cell function, the mechanisms by which cross-linking proteins coordinate actin and microtubule activities remain poorly understood. In particular, it is unknown how the distinct mechanical properties of different actin architectures modulate the outcome of actin-microtubule interactions. To address this question, we engineered the protein TipAct, which links growing microtubule ends via end-binding proteins to actin filaments. We show that growing microtubules can be captured and guided by stiff actin bundles, leading to global actin-microtubule alignment. Conversely, growing microtubule ends can transport, stretch and bundle individual actin filaments, thereby globally defining actin filament organization. Our results provide a physical basis to understand actin-microtubule cross-talk, and reveal that a simple cross-linker can enable a mechanical feedback between actin and microtubule organization that is relevant to diverse biological contexts.
Figures



References
-
- Rodriguez O. C. et al. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat. Rev. Mol. Cell Biol. 5, 599–609 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

