Metal preferences and metallation
- PMID: 25160626
- PMCID: PMC4192464
- DOI: 10.1074/jbc.R114.588145
Metal preferences and metallation
Abstract
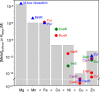
The metal binding preferences of most metalloproteins do not match their metal requirements. Thus, metallation of an estimated 30% of metalloenzymes is aided by metal delivery systems, with ∼ 25% acquiring preassembled metal cofactors. The remaining ∼ 70% are presumed to compete for metals from buffered metal pools. Metallation is further aided by maintaining the relative concentrations of these pools as an inverse function of the stabilities of the respective metal complexes. For example, magnesium enzymes always prefer to bind zinc, and these metals dominate the metalloenzymes without metal delivery systems. Therefore, the buffered concentration of zinc is held at least a million-fold below magnesium inside most cells.
Keywords: Copper; Iron; Irving-Williams Series; Manganese; Metal Sensors; Metallochaperone; Metalloenzymes; Nickel; Polydisperse Buffer; Zinc.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


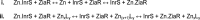
References
-
- Irving H., Williams R. J. (1948) Order of stability of metal complexes. Nature 162, 746–747
-
- Johnson D. A., Nelson P. G. (1995) Factors determining the ligand field stabilization energies of the hexaaqua 2+ complexes of the first transition series and the Irving-Williams order. Inorg. Chem. 34, 5666–5671 - PubMed
-
- Schalk I. J., Yue W. W., Buchanan S. K. (2004) Recognition of iron-free siderophores by TonB-dependent iron transporters. Mol. Microbiol. 54, 14–22 - PubMed
-
- Parker Siburt C. J., Mietzner T. A., Crumbliss A. L. (2012) FbpA — a bacterial transferrin with more to offer. Biochim. Biophys. Acta 1820, 379–392 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

