Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans
- PMID: 25161212
- PMCID: PMC4224173
- DOI: 10.1534/genetics.114.169730
Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans
Abstract
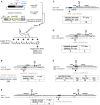
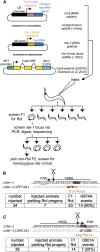
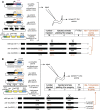
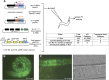
Facilitated by recent advances using CRISPR/Cas9, genome editing technologies now permit custom genetic modifications in a wide variety of organisms. Ideally, modified animals could be both efficiently made and easily identified with minimal initial screening and without introducing exogenous sequence at the locus of interest or marker mutations elsewhere. To this end, we describe a coconversion strategy, using CRISPR/Cas9 in which screening for a dominant phenotypic oligonucleotide-templated conversion event at one locus can be used to enrich for custom modifications at another unlinked locus. After the desired mutation is identified among the F1 progeny heterozygous for the dominant marker mutation, F2 animals that have lost the marker mutation are picked to obtain the desired mutation in an unmarked genetic background. We have developed such a coconversion strategy for Caenorhabditis elegans, using a number of dominant phenotypic markers. Examining the coconversion at a second (unselected) locus of interest in the marked F1 animals, we observed that 14-84% of screened animals showed homologous recombination. By reconstituting the unmarked background through segregation of the dominant marker mutation at each step, we show that custom modification events can be carried out recursively, enabling multiple mutant animals to be made. While our initial choice of a coconversion marker [rol-6(su1006)] was readily applicable in a single round of coconversion, the genetic properties of this locus were not optimal in that CRISPR-mediated deletion mutations at the unselected rol-6 locus can render a fraction of coconverted strains recalcitrant to further rounds of similar mutagenesis. An optimal marker in this sense would provide phenotypic distinctions between the desired mutant/+ class and alternative +/+, mutant/null, null/null, and null/+ genotypes. Reviewing dominant alleles from classical C. elegans genetics, we identified one mutation in dpy-10 and one mutation in sqt-1 that meet these criteria and demonstrate that these too can be used as effective conversion markers. Coconversion was observed using a variety of donor molecules at the second (unselected) locus, including oligonucleotides, PCR products, and plasmids. We note that the coconversion approach described here could be applied in any of the variety of systems where suitable coconversion markers can be identified from previous intensive genetic analyses of gain-of-function alleles.
Keywords: CRISPR/Cas9; coconversion; dpy-10; oligonucleotide-mediated homologous recombination; rde-1.
Copyright © 2014 by the Genetics Society of America.
Figures

References
-
- Chen, P. A., and E. Jorgensen, 2013 Trimeric G-proteins converge on a novel ion channel. Ph.D. Thesis, University of Utah, Salt Lake City
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

