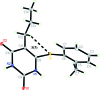
6-[(2-Methyl-phen-yl)sulfan-yl]-5-propyl-pyrimidine-2,4(1H,3H)-dione
- PMID: 25161558
- PMCID: PMC4120546
- DOI: 10.1107/S1600536814013269
6-[(2-Methyl-phen-yl)sulfan-yl]-5-propyl-pyrimidine-2,4(1H,3H)-dione
Abstract
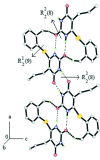
In the title pyrimidine-2,4-dione derivative, C14H16N2O2S, the dihedral angle between the six-membered rings is 77.81 (10)°. The mol-ecule is twisted about the Cp-S (p = pyrimidine) bond, with a C-S-C-N torsion angle of -59.01 (17)°. An intramolecular C-H⋯S hydrogen bond generates an S(5) ring motif. In the crystal, bifurcated acceptor N-H⋯O and C-H⋯O hydrogen bonds generate inversion-related dimers incorporating R 2 (1)(9) and R 2 (2)(8) loops. These dimers are connected into a chain extending along the a-axis direction by a second pair of inversion-related N-H⋯O hydrogen bonds, forming another R 2 (2)(8) loop. The crystal structure is further stabilized by weak inter-molecular C-H⋯π inter-actions, generating a three-dimensional network.
Figures
References
-
- Al-Abdullah, E. S., Al-Obaid, A. M., Al-Deeb, O. A., Habib, E. E. & El-Emam, A. A. (2011). Eur. J. Med. Chem 46, 4642–4647. - PubMed
-
- Al-Abdullah, E. S., Al-Turkistani, A. A., Al-Deeb, O. A., El-Brollosy, N. R., Habib, E. E. & El-Emam, A. A. (2014). Drug Res 64, 31–39. - PubMed
-
- Al-Deeb, O. A., Al-Turkistani, A. A., El-Brollosy, N. R., Habib, E. E. & El-Emam, A. A. (2013). Heterocycl. Commun 19, 411–419.
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–S19.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous