Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens
- PMID: 25161649
- PMCID: PMC4130309
- DOI: 10.3389/fmicb.2014.00405
Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens
Abstract
With increasing numbers of antibiotic-resistant pathogens all over the world there is a pressing need for strategies that are capable of inactivating biofilm-state pathogens with less potential of developing resistances in pathogens. Antimicrobial strategies of that kind are especially needed in dentistry in order to avoid the usage of antibiotics for treatment of periodontal, endodontic or mucosal topical infections caused by bacterial or yeast biofilms. One possible option could be the antimicrobial photodynamic therapy (aPDT), whereby the lethal effect of aPDT is based on the principle that visible light activates a photosensitizer (PS), leading to the formation of reactive oxygen species, e.g., singlet oxygen, which induce phototoxicity immediately during illumination. Many compounds have been described as potential PS for aPDT against bacterial and yeast biofilms so far, but conflicting results have been reported. Therefore, the aim of the present review is to outline the actual state of the art regarding the potential of aPDT for inactivation of biofilms formed in vitro with a main focus on those formed by oral key pathogens and structured regarding the distinct types of PS.
Keywords: aPDT; antibiotic resistance; antimicrobial; biofilm; oral; photodynamic.
Figures

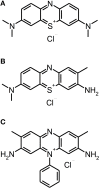

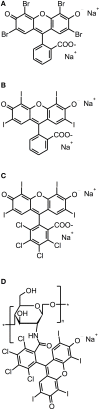
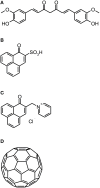
References
-
- Al-Ahmad A., Ameen H., Pelz K., Karygianni L., Wittmer A., Anderson A. C., et al. (2014). Antibiotic resistance and capacity for biofilm formation of different bacteria isolated from endodontic infections associated with root-filled teeth. J. Endod. 40, 223–230 10.1016/j.joen.2013.07.023 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

