Microtubule-associated protein Mdp3 promotes breast cancer growth and metastasis
- PMID: 25161703
- PMCID: PMC4143944
- DOI: 10.7150/thno.9727
Microtubule-associated protein Mdp3 promotes breast cancer growth and metastasis
Erratum in
-
Erratum: Microtubule-Associated Protein Mdp3 Promotes Breast Cancer Growth and Metastasis: Erratum.Theranostics. 2020 Apr 12;10(12):5527. doi: 10.7150/thno.45566. eCollection 2020. Theranostics. 2020. PMID: 32373226 Free PMC article.
Abstract
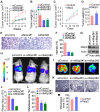
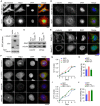
Breast cancer is the most prevalent cancer in women worldwide with a high mortality rate, and the identification of new biomarkers and targets for this disease is greatly needed. Here we present evidence that microtubule-associated protein (MAP) 7 domain-containing protein 3 (Mdp3) is highly expressed in clinical samples and cell lines of breast cancer. The expression of Mdp3 correlates with clinicopathological parameters indicating breast cancer malignancy. In addition, Mdp3 promotes breast cancer cell proliferation and motility in vitro and stimulates breast cancer growth and metastasis in mice. Mechanistic studies reveal that γ-tubulin interacts with and recruits Mdp3 to the centrosome and that the centrosomal localization of Mdp3 is required for its activity to promote breast cancer cell proliferation and motility. These findings suggest a critical role for Mdp3 in the growth and metastasis of breast cancer and may have important implications for the management of this disease.
Keywords: breast cancer; cell motility; cell proliferation; growth; metastasis..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Mdp3 is a novel microtubule-binding protein that regulates microtubule assembly and stability.Cell Cycle. 2011 Nov 15;10(22):3929-37. doi: 10.4161/cc.10.22.18106. Epub 2011 Nov 15. Cell Cycle. 2011. PMID: 22142902
-
Microtubule stabilization by Mdp3 is partially attributed to its modulation of HDAC6 in addition to its association with tubulin and microtubules.PLoS One. 2014 Mar 10;9(3):e90932. doi: 10.1371/journal.pone.0090932. eCollection 2014. PLoS One. 2014. PMID: 24614595 Free PMC article.
-
Centrosomal Protein 70 Is a Mediator of Paclitaxel Sensitivity.Int J Mol Sci. 2017 Jun 20;18(6):1267. doi: 10.3390/ijms18061267. Int J Mol Sci. 2017. PMID: 28632150 Free PMC article.
-
Discovery of Centrosomal Protein 70 as an Important Player in the Development and Progression of Breast Cancer.Am J Pathol. 2017 Mar;187(3):679-688. doi: 10.1016/j.ajpath.2016.11.005. Epub 2017 Jan 5. Am J Pathol. 2017. PMID: 28063737
-
Oncogenic function of microtubule end-binding protein 1 in breast cancer.J Pathol. 2010 Feb;220(3):361-9. doi: 10.1002/path.2662. J Pathol. 2010. PMID: 19967727
Cited by
-
STAT3 association with microtubules and its activation are independent of HDAC6 activity.DNA Cell Biol. 2015 Apr;34(4):290-5. doi: 10.1089/dna.2014.2713. Epub 2015 Jan 26. DNA Cell Biol. 2015. PMID: 25621430 Free PMC article.
-
Cytoskeletal Remodeling in Cancer.Biology (Basel). 2020 Nov 7;9(11):385. doi: 10.3390/biology9110385. Biology (Basel). 2020. PMID: 33171868 Free PMC article. Review.
-
A Decision Tree Based Classifier to Analyze Human Ovarian Cancer cDNA Microarray Datasets.J Med Syst. 2016 Jan;40(1):21. doi: 10.1007/s10916-015-0361-9. Epub 2015 Nov 3. J Med Syst. 2016. PMID: 26531754
-
Identification of novel microtubule-binding proteins by taxol-mediated microtubule stabilization and mass spectrometry analysis.Thorac Cancer. 2015 Sep;6(5):649-54. doi: 10.1111/1759-7714.12284. Epub 2015 Jun 12. Thorac Cancer. 2015. PMID: 26445615 Free PMC article.
-
CYLD Regulates Noscapine Activity in Acute Lymphoblastic Leukemia via a Microtubule-Dependent Mechanism.Theranostics. 2015 Mar 2;5(7):656-66. doi: 10.7150/thno.10844. eCollection 2015. Theranostics. 2015. PMID: 25897332 Free PMC article.
References
-
- Jemal A, Bray F, Center MM. et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Lu J, Steeg PS, Price JE. et al. Breast cancer metastasis: challenges and opportunities. Cancer Res. 2009;69:4951–3. - PubMed
-
- Stern HM. Improving treatment of HER2-positive cancers: opportunities and challenges. Sci Transl Med. 2012;4:127rv2. - PubMed
-
- Amos LA, Schlieper D. Microtubules and maps. Adv Protein Chem. 2005;71:257–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

