Pyrene-modified PNAs: Stacking interactions and selective excimer emission in PNA2DNA triplexes
- PMID: 25161706
- PMCID: PMC4142857
- DOI: 10.3762/bjoc.10.154
Pyrene-modified PNAs: Stacking interactions and selective excimer emission in PNA2DNA triplexes
Abstract
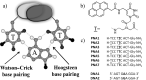
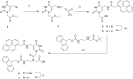
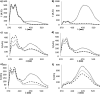
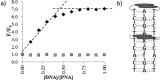
Pyrene derivatives can be incorporated into nucleic acid analogs in order to obtain switchable probes or supramolecular architectures. In this paper, peptide nucleic acids (PNAs) containing 1 to 3 1-pyreneacetic acid units (PNA1-6) with a sequence with prevalence of pyrimidine bases, complementary to cystic fibrosis W1282X point mutation were synthesized. These compounds showed sequence-selective switch-on of pyrene excimer emission in the presence of target DNA, due to PNA2DNA triplex formation, with stability depending on the number and positioning of the pyrene units along the chain. An increase in triplex stability and a very high mismatch-selectivity, derived from combined stacking and base-pairing interactions, were found for PNA2, bearing two distant pyrene units.
Keywords: PNA; SNP recognition; modified nucleobase; nucleic acids; pyrene excimer; triplex stabilization.
Figures

Similar articles
-
Incorporation of thio-pseudoisocytosine into triplex-forming peptide nucleic acids for enhanced recognition of RNA duplexes.Nucleic Acids Res. 2014 Apr;42(6):4008-18. doi: 10.1093/nar/gkt1367. Epub 2014 Jan 13. Nucleic Acids Res. 2014. PMID: 24423869 Free PMC article.
-
Metal Coordination to Ligand-Modified Peptide Nucleic Acid Triplexes.Inorg Chem. 2018 Jun 18;57(12):6865-6872. doi: 10.1021/acs.inorgchem.8b00442. Epub 2018 May 30. Inorg Chem. 2018. PMID: 29845860
-
General Recognition of U-G, U-A, and C-G Pairs by Double-Stranded RNA-Binding PNAs Incorporated with an Artificial Nucleobase.Biochemistry. 2019 Mar 12;58(10):1319-1331. doi: 10.1021/acs.biochem.8b01313. Epub 2019 Mar 1. Biochemistry. 2019. PMID: 30775913
-
Pyrene excimer nucleic acid probes for biomolecule signaling.J Biomed Nanotechnol. 2009 Oct;5(5):495-504. doi: 10.1166/jbn.2009.1074. J Biomed Nanotechnol. 2009. PMID: 20201423 Review.
-
RNA triplexes: from structural principles to biological and biotech applications.Wiley Interdiscip Rev RNA. 2015 Jan-Feb;6(1):111-28. doi: 10.1002/wrna.1261. Epub 2014 Aug 22. Wiley Interdiscip Rev RNA. 2015. PMID: 25146348 Review.
Cited by
-
Pyrene-nucleobase conjugates: synthesis, oligonucleotide binding and confocal bioimaging studies.Beilstein J Org Chem. 2017 Nov 28;13:2521-2534. doi: 10.3762/bjoc.13.249. eCollection 2017. Beilstein J Org Chem. 2017. PMID: 29259662 Free PMC article.
-
Antibacterial Peptide Nucleic Acids-Facts and Perspectives.Molecules. 2020 Jan 28;25(3):559. doi: 10.3390/molecules25030559. Molecules. 2020. PMID: 32012929 Free PMC article. Review.
-
Novel nicotinoid structures for covalent modification of wood: an environmentally friendly way for its protection against insects.RSC Adv. 2020 Apr 21;10(27):15726-15733. doi: 10.1039/d0ra02071k. eCollection 2020 Apr 21. RSC Adv. 2020. PMID: 35493663 Free PMC article.
-
Fluorogenic PNA probes.Beilstein J Org Chem. 2018 Jan 29;14:253-281. doi: 10.3762/bjoc.14.17. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 29507634 Free PMC article. Review.
References
-
- Nielsen P E. Peptide Nucleic Acids: Protocols and Applications. Norwich, UK: Horizon Bioscience; 2004.
-
- Bertucci A, Manicardi A, Corradini R. Advanced Molecular Probes for Sequence-Specific DNA Recognition. In: Spoto G, Corradini R, editors. Detection of non-amplified Genomic DNA. Dordrecht, The Netherlands: Springer; 2012. pp. 89–124. - DOI
-
- Wittung P, Nielsen P E, Nordén B. J Am Chem Soc. 1996;118:7049–7054. doi: 10.1021/ja960521f. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
