Multivalent scaffolds induce galectin-3 aggregation into nanoparticles
- PMID: 25161713
- PMCID: PMC4142985
- DOI: 10.3762/bjoc.10.162
Multivalent scaffolds induce galectin-3 aggregation into nanoparticles
Abstract
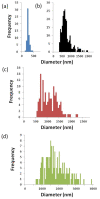
Galectin-3 meditates cell surface glycoprotein clustering, cross linking, and lattice formation. In cancer biology, galectin-3 has been reported to play a role in aggregation processes that lead to tumor embolization and survival. Here, we show that lactose-functionalized dendrimers interact with galectin-3 in a multivalent fashion to form aggregates. The glycodendrimer-galectin aggregates were characterized by dynamic light scattering and fluorescence microscopy methodologies and were found to be discrete particles that increased in size as the dendrimer generation was increased. These results show that nucleated aggregation of galectin-3 can be regulated by the nucleating polymer and provide insights that improve the general understanding of the binding and function of sugar-binding proteins.
Keywords: dendrimers; galectin-3; glycodendrimers; multivalency; multivalent glycosylation; protein aggregation.
Figures

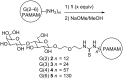



Similar articles
-
Glycodendrimers and Modified ELISAs: Tools to Elucidate Multivalent Interactions of Galectins 1 and 3.Molecules. 2015 Apr 20;20(4):7059-96. doi: 10.3390/molecules20047059. Molecules. 2015. PMID: 25903363 Free PMC article.
-
Glycodendrimers: tools to explore multivalent galectin-1 interactions.Beilstein J Org Chem. 2015 May 12;11:739-47. doi: 10.3762/bjoc.11.84. eCollection 2015. Beilstein J Org Chem. 2015. PMID: 26124876 Free PMC article.
-
Lactose-functionalized dendrimers arbitrate the interaction of galectin-3/MUC1 mediated cancer cellular aggregation.Chembiochem. 2014 Sep 22;15(14):2106-12. doi: 10.1002/cbic.201402134. Epub 2014 Aug 19. Chembiochem. 2014. PMID: 25138772 Free PMC article.
-
The Role of Galectin-1 in Cancer Progression, and Synthetic Multivalent Systems for the Study of Galectin-1.Int J Mol Sci. 2016 Sep 16;17(9):1566. doi: 10.3390/ijms17091566. Int J Mol Sci. 2016. PMID: 27649167 Free PMC article. Review.
-
Glycodendrimers: novel glycotope isosteres unmasking sugar coding. case study with T-antigen markers from breast cancer MUC1 glycoprotein.J Biotechnol. 2002 May;90(3-4):291-309. doi: 10.1016/s1389-0352(01)00065-4. J Biotechnol. 2002. PMID: 12071230 Review.
Cited by
-
Glycodendrimers and Modified ELISAs: Tools to Elucidate Multivalent Interactions of Galectins 1 and 3.Molecules. 2015 Apr 20;20(4):7059-96. doi: 10.3390/molecules20047059. Molecules. 2015. PMID: 25903363 Free PMC article.
-
Multivalent Lactose-Ferrocene Conjugates Based on Poly (Amido Amine) Dendrimers and Gold Nanoparticles as Electrochemical Probes for Sensing Galectin-3.Nanomaterials (Basel). 2020 Jan 24;10(2):203. doi: 10.3390/nano10020203. Nanomaterials (Basel). 2020. PMID: 31991555 Free PMC article.
-
Effects of linker and liposome anchoring on lactose-functionalized glycomacromolecules as multivalent ligands for binding galectin-3.RSC Adv. 2019 Jul 29;9(41):23484-23497. doi: 10.1039/c9ra05497a. eCollection 2019 Jul 29. RSC Adv. 2019. PMID: 35530592 Free PMC article.
-
Crystallization of a human galectin-3 variant with two ordered segments in the shortened N-terminal tail.Sci Rep. 2018 Jun 29;8(1):9835. doi: 10.1038/s41598-018-28235-x. Sci Rep. 2018. PMID: 29959397 Free PMC article.
-
Biotinylated N-Acetyllactosamine- and N,N-Diacetyllactosamine-Based Oligosaccharides as Novel Ligands for Human Galectin-3.Bioengineering (Basel). 2017 Apr 5;4(2):31. doi: 10.3390/bioengineering4020031. Bioengineering (Basel). 2017. PMID: 28952509 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
