Influence of perylenediimide-pyrene supramolecular interactions on the stability of DNA-based hybrids: Importance of electrostatic complementarity
- PMID: 25161715
- PMCID: PMC4142898
- DOI: 10.3762/bjoc.10.164
Influence of perylenediimide-pyrene supramolecular interactions on the stability of DNA-based hybrids: Importance of electrostatic complementarity
Abstract
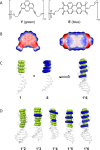
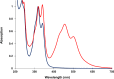
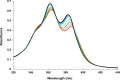
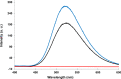
Aromatic π-π stacking interactions are ubiquitous in nature, medicinal chemistry and materials sciences. They play a crucial role in the stacking of nucleobases, thus stabilising the DNA double helix. The following paper describes a series of chimeric DNA-polycyclic aromatic hydrocarbon (PAH) hybrids. The PAH building blocks are electron-rich pyrene and electron-poor perylenediimide (PDI), and were incorporated into complementary DNA strands. The hybrids contain different numbers of pyrene-PDI interactions that were found to directly influence duplex stability. As the pyrene-PDI ratio approaches 1:1, the stability of the duplexes increases with an average value of 7.5 °C per pyrene-PDI supramolecular interaction indicating the importance of electrostatic complementarity for aromatic π-π stacking interactions.
Keywords: DNA; hybridization; nucleic acids; perylenediimide; pyrene.
Figures



Similar articles
-
Role of electrostatic complementarity between perylenediimide and porphyrin in highly stabilized GNA.Mater Sci Eng C Mater Biol Appl. 2017 Jan 1;70(Pt 2):1156-1162. doi: 10.1016/j.msec.2016.03.111. Epub 2016 Apr 10. Mater Sci Eng C Mater Biol Appl. 2017. PMID: 27772717
-
The 5-Me of thyminyl (T) interaction with the neighboring nucleobases dictate the relative stability of isosequential DNA-RNA hybrid duplexes.Org Biomol Chem. 2005 Nov 7;3(21):3911-5. doi: 10.1039/b511139k. Epub 2005 Sep 29. Org Biomol Chem. 2005. PMID: 16240008
-
Free energy profiles of base flipping in intercalative polycyclic aromatic hydrocarbon-damaged DNA duplexes: energetic and structural relationships to nucleotide excision repair susceptibility.Chem Res Toxicol. 2013 Jul 15;26(7):1115-25. doi: 10.1021/tx400156a. Epub 2013 Jul 2. Chem Res Toxicol. 2013. PMID: 23758590 Free PMC article.
-
Spectroscopic properties of pyrene-containing DNA mimics.Bioorg Med Chem. 2008 Jan 1;16(1):27-33. doi: 10.1016/j.bmc.2007.04.052. Epub 2007 May 3. Bioorg Med Chem. 2008. PMID: 17512737
-
Aromatic Base Stacking in DNA: From ab initio Calculations to Molecular Dynamics Simulations.J Biomol Struct Dyn. 2000;17 Suppl 1:1-24. doi: 10.1080/07391102.2000.10506597. J Biomol Struct Dyn. 2000. PMID: 22607400 Review.
Cited by
-
Recent Advances in Nucleic Acid Targeting Probes and Supramolecular Constructs Based on Pyrene-Modified Oligonucleotides.Molecules. 2017 Nov 30;22(12):2108. doi: 10.3390/molecules22122108. Molecules. 2017. PMID: 29189716 Free PMC article. Review.
-
Tailored Tolane-Perfluorotolane Assembly as Supramolecular Base Pair Replacement in DNA.Angew Chem Int Ed Engl. 2023 Jan 2;62(1):e202214456. doi: 10.1002/anie.202214456. Epub 2022 Dec 1. Angew Chem Int Ed Engl. 2023. PMID: 36344446 Free PMC article.
-
Amphiphilic DNA Organic Hybrids: Functional Materials in Nanoscience and Potential Application in Biomedicine.Int J Mol Sci. 2018 Aug 3;19(8):2283. doi: 10.3390/ijms19082283. Int J Mol Sci. 2018. PMID: 30081520 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
