Triazol-substituted titanocenes by strain-driven 1,3-dipolar cycloadditions
- PMID: 25161720
- PMCID: PMC4142980
- DOI: 10.3762/bjoc.10.169
Triazol-substituted titanocenes by strain-driven 1,3-dipolar cycloadditions
Abstract
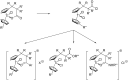
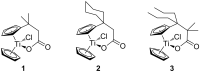
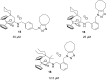
An operationally simple, convenient, and mild strategy for the synthesis of triazole-substituted titanocenes via strain-driven 1,3-dipolar cycloadditions between azide-functionalized titanocenes and cyclooctyne has been developed. It features the first synthesis of titanocenes containing azide groups. These compounds constitute 'second-generation' functionalized titanocene building blocks for further synthetic elaboration. Our synthesis is modular and large numbers of the complexes can in principle be prepared in short periods of time. Some of the triazole-substituted titanocenes display high cyctotoxic activity against BJAB cells. Comparison of the most active complexes allows the identification of structural features essential for biological activity.
Keywords: azides; click-chemistry; cycloadditions; cytotoxicity; titanocenes.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
