Chemistry of polyhalogenated nitrobutadienes, 14: Efficient synthesis of functionalized (Z)-2-allylidenethiazolidin-4-ones
- PMID: 25161721
- PMCID: PMC4142838
- DOI: 10.3762/bjoc.10.170
Chemistry of polyhalogenated nitrobutadienes, 14: Efficient synthesis of functionalized (Z)-2-allylidenethiazolidin-4-ones
Abstract
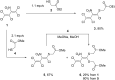
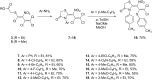
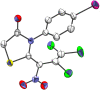
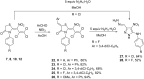
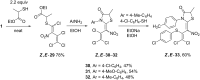
The reaction of mercaptoacetic acid esters with pentachloro-2-nitro-1,3-butadiene (1) provides an appropriate precursor for the synthesis of special thiazolidin-4-ones. Applying different anilines as the second constituent for the requisite cyclization step, a series of (Z)-2-allylidenethiazolidin-4-ones was obtained in yields up to 81%. Some subsequent reactions have been examined too, such as the formation of perfunctionalized 1H-pyrazoles upon treatment with hydrazine. Thiazolidinones are as well known for their physiological activities as for their application in optoelectronics.
Keywords: 1H-pyrazoles; 2-nitroperchlorobutadiene; atropisomers; cyclization; thiazolidin-4-ones.
Figures

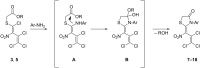


Similar articles
-
A New Way to 2,3,4-Trisubstituted Benzo[h]quinolines: Synthesis, Consecutive Reactions and Cellular Activities †.Molecules. 2023 Mar 8;28(6):2479. doi: 10.3390/molecules28062479. Molecules. 2023. PMID: 36985452 Free PMC article.
-
Chemistry of polyhalogenated nitrobutadienes, 17: Efficient synthesis of persubstituted chloroquinolinyl-1H-pyrazoles and evaluation of their antimalarial, anti-SARS-CoV-2, antibacterial, and cytotoxic activities.Beilstein J Org Chem. 2022 May 9;18:524-532. doi: 10.3762/bjoc.18.54. eCollection 2022. Beilstein J Org Chem. 2022. PMID: 35615535 Free PMC article.
-
Mechanisms of the reaction between polyhalogenated nitrobutadienes and electron-deficient anilines: computational modeling.J Org Chem. 2014 Mar 7;79(5):2123-38. doi: 10.1021/jo402858j. Epub 2014 Feb 25. J Org Chem. 2014. PMID: 24533665
-
Chemistry of polyhalogenated nitrobutadienes, 10: Synthesis of highly functionalized heterocycles with a rigid 6-amino-3-azabicyclo[3.1.0]hexane moiety.Beilstein J Org Chem. 2012;8:621-8. doi: 10.3762/bjoc.8.69. Epub 2012 Apr 23. Beilstein J Org Chem. 2012. PMID: 22563360 Free PMC article.
-
Efficient sonochemical synthesis of thiazolidinones from piperonilamine.Ultrason Sonochem. 2011 Jan;18(1):65-7. doi: 10.1016/j.ultsonch.2010.07.008. Epub 2010 Jul 27. Ultrason Sonochem. 2011. PMID: 20724206
Cited by
-
Polyhalonitrobutadienes as Versatile Building Blocks for the Biotargeted Synthesis of Substituted N-Heterocyclic Compounds.Molecules. 2020 Jun 21;25(12):2863. doi: 10.3390/molecules25122863. Molecules. 2020. PMID: 32575902 Free PMC article.
-
5-Ene-4-thiazolidinones - An efficient tool in medicinal chemistry.Eur J Med Chem. 2017 Nov 10;140:542-594. doi: 10.1016/j.ejmech.2017.09.031. Epub 2017 Sep 20. Eur J Med Chem. 2017. PMID: 28987611 Free PMC article. Review.
-
A New Way to 2,3,4-Trisubstituted Benzo[h]quinolines: Synthesis, Consecutive Reactions and Cellular Activities †.Molecules. 2023 Mar 8;28(6):2479. doi: 10.3390/molecules28062479. Molecules. 2023. PMID: 36985452 Free PMC article.
References
-
- Kaberdin R V, Potkin V I, Zapol’skii V A. Russ Chem Rev. 1997;66:827–842. doi: 10.1070/RC1997v066n10ABEH000310. - DOI
-
- Zapol’skii V A, Namyslo J C, Adam A E, Kaufmann D E. Heterocycles. 2004;63:1281–1298. doi: 10.3987/COM-04-10020. - DOI
-
- Zapol’skii V A, Nutz E, Namyslo J C, Adam A E, Kaufmann D E. Synthesis. 2006:2927–2933. doi: 10.1055/s-2006-950187. - DOI
-
- Zapol’skii V A, Namyslo J C, Blaschkowski B, Kaufmann D E. Synlett. 2006:3464–3468. doi: 10.1055/s-2006-956490. - DOI
-
- Zapol’skii V A, Namyslo J C, Gjikaj M, Kaufmann D E. ARKIVOC. 2007;(i):76–93.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
