The chemoenzymatic synthesis of clofarabine and related 2'-deoxyfluoroarabinosyl nucleosides: the electronic and stereochemical factors determining substrate recognition by E. coli nucleoside phosphorylases
- PMID: 25161724
- PMCID: PMC4142866
- DOI: 10.3762/bjoc.10.173
The chemoenzymatic synthesis of clofarabine and related 2'-deoxyfluoroarabinosyl nucleosides: the electronic and stereochemical factors determining substrate recognition by E. coli nucleoside phosphorylases
Abstract
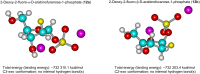
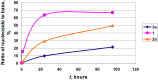
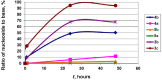
Two approaches to the synthesis of 2-chloro-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)adenine (1, clofarabine) were studied. The first approach consists in the chemical synthesis of 2-deoxy-2-fluoro-α-D-arabinofuranose-1-phosphate (12a, (2F)Ara-1P) via three step conversion of 1,3,5-tri-O-benzoyl-2-deoxy-2-fluoro-α-D-arabinofuranose (9) into the phosphate 12a without isolation of intermediary products. Condensation of 12a with 2-chloroadenine catalyzed by the recombinant E. coli purine nucleoside phosphorylase (PNP) resulted in the formation of clofarabine in 67% yield. The reaction was also studied with a number of purine bases (2-aminoadenine and hypoxanthine), their analogues (5-aza-7-deazaguanine and 8-aza-7-deazahypoxanthine) and thymine. The results were compared with those of a similar reaction with α-D-arabinofuranose-1-phosphate (13a, Ara-1P). Differences of the reactivity of various substrates were analyzed by ab initio calculations in terms of the electronic structure (natural purines vs analogues) and stereochemical features ((2F)Ara-1P vs Ara-1P) of the studied compounds to determine the substrate recognition by E. coli nucleoside phosphorylases. The second approach starts with the cascade one-pot enzymatic transformation of 2-deoxy-2-fluoro-D-arabinose into the phosphate 12a, followed by its condensation with 2-chloroadenine thereby affording clofarabine in ca. 48% yield in 24 h. The following recombinant E. coli enzymes catalyze the sequential conversion of 2-deoxy-2-fluoro-D-arabinose into the phosphate 12a: ribokinase (2-deoxy-2-fluoro-D-arabinofuranose-5-phosphate), phosphopentomutase (PPN; no 1,6-diphosphates of D-hexoses as co-factors required) (12a), and finally PNP. The substrate activities of D-arabinose, D-ribose and D-xylose in the similar cascade syntheses of the relevant 2-chloroadenine nucleosides were studied and compared with the activities of 2-deoxy-2-fluoro-D-arabinose. As expected, D-ribose exhibited the best substrate activity [90% yield of 2-chloroadenosine (8) in 30 min], D-arabinose reached an equilibrium at a concentration of ca. 1:1 of a starting base and the formed 2-chloro-9-(β-D-arabinofuranosyl)adenine (6) in 45 min, the formation of 2-chloro-9-(β-D-xylofuranosyl)adenine (7) proceeded very slowly attaining ca. 8% yield in 48 h.
Keywords: chemoenzymatic synthesis; clofarabine; nucleoside phosphorylases; phosphopentomutase; recombinant E. coli ribokinase.
Figures






Similar articles
-
Multi-Enzymatic Cascades in the Synthesis of Modified Nucleosides: Comparison of the Thermophilic and Mesophilic Pathways.Biomolecules. 2021 Apr 16;11(4):586. doi: 10.3390/biom11040586. Biomolecules. 2021. PMID: 33923608 Free PMC article.
-
Enzymatic synthesis and phosphorolysis of 4(2)-thioxo- and 6(5)-azapyrimidine nucleosides by E. coli nucleoside phosphorylases.Beilstein J Org Chem. 2016 Dec 1;12:2588-2601. doi: 10.3762/bjoc.12.254. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 28144328 Free PMC article.
-
A new synthesis of 9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)guanine (AraF-G).Nucleosides Nucleotides Nucleic Acids. 2003 May-Aug;22(5-8):1339-42. doi: 10.1081/NCN-120022960. Nucleosides Nucleotides Nucleic Acids. 2003. PMID: 14565413
-
Strained Conformations of Nucleosides in Active Sites of Nucleoside Phosphorylases.Biomolecules. 2020 Apr 5;10(4):552. doi: 10.3390/biom10040552. Biomolecules. 2020. PMID: 32260512 Free PMC article. Review.
-
Biosynthesis of D-arabinose in mycobacteria - a novel bacterial pathway with implications for antimycobacterial therapy.FEBS J. 2008 Jun;275(11):2691-711. doi: 10.1111/j.1742-4658.2008.06395.x. Epub 2008 Apr 15. FEBS J. 2008. PMID: 18422659 Review.
Cited by
-
Enzymatic Transglycosylation Features in Synthesis of 8-Aza-7-Deazapurine Fleximer Nucleosides by Recombinant E. coli PNP: Synthesis and Structure Determination of Minor Products.Biomolecules. 2024 Jul 4;14(7):798. doi: 10.3390/biom14070798. Biomolecules. 2024. PMID: 39062512 Free PMC article.
-
Enzymatic Synthesis of 2-Chloropurine Arabinonucleosides with Chiral Amino Acid Amides at the C6 Position and an Evaluation of Antiproliferative Activity In Vitro.Int J Mol Sci. 2023 Mar 25;24(7):6223. doi: 10.3390/ijms24076223. Int J Mol Sci. 2023. PMID: 37047197 Free PMC article.
-
Spectral Unmixing-Based Reaction Monitoring of Transformations between Nucleosides and Nucleobases.Chembiochem. 2020 Sep 14;21(18):2604-2610. doi: 10.1002/cbic.202000204. Epub 2020 Jun 18. Chembiochem. 2020. PMID: 32324971 Free PMC article.
-
Multi-Enzymatic Cascades in the Synthesis of Modified Nucleosides: Comparison of the Thermophilic and Mesophilic Pathways.Biomolecules. 2021 Apr 16;11(4):586. doi: 10.3390/biom11040586. Biomolecules. 2021. PMID: 33923608 Free PMC article.
-
An Expedient Synthesis of Flexible Nucleosides through Enzymatic Glycosylation of Proximal and Distal Fleximer Bases.Chembiochem. 2020 May 15;21(10):1412-1417. doi: 10.1002/cbic.201900714. Epub 2020 Feb 27. Chembiochem. 2020. PMID: 31899839 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
