Model systems for studying cell adhesion and biomimetic actin networks
- PMID: 25161853
- PMCID: PMC4142981
- DOI: 10.3762/bjnano.5.131
Model systems for studying cell adhesion and biomimetic actin networks
Abstract
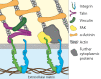
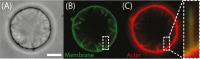
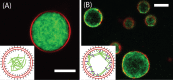
Many cellular processes, such as migration, proliferation, wound healing and tumor progression are based on cell adhesion. Amongst different cell adhesion molecules, the integrin receptors play a very significant role. Over the past decades the function and signalling of various such integrins have been studied by incorporating the proteins into lipid membranes. These proteolipid structures lay the foundation for the development of artificial cells, which are able to adhere to substrates. To build biomimetic models for studying cell shape and spreading, actin networks can be incorporated into lipid vesicles, too. We here review the mechanisms of integrin-mediated cell adhesion and recent advances in the field of minimal cells towards synthetic adhesion. We focus on reconstituting integrins into lipid structures for mimicking cell adhesion and on the incorporation of actin networks and talin into model cells.
Keywords: actin network; cell adhesion; giant unilamellar vesicle; integrin; lipid bilayer; protein reconstitution; synthetic cell; talin.
Figures




Similar articles
-
Studying actin-induced cell shape changes using Giant Unilamellar Vesicles and reconstituted actin networks.Biochem Soc Trans. 2022 Oct 31;50(5):1527-1539. doi: 10.1042/BST20220900. Biochem Soc Trans. 2022. PMID: 36111807 Free PMC article. Review.
-
In Vitro Reconstitution of the Actin Cytoskeleton Inside Giant Unilamellar Vesicles.J Vis Exp. 2022 Aug 25;(186). doi: 10.3791/64026. J Vis Exp. 2022. PMID: 36094272
-
Talin and kindlin cooperate to control the density of integrin clusters.J Cell Sci. 2023 Apr 15;136(8):jcs260746. doi: 10.1242/jcs.260746. Epub 2023 Apr 21. J Cell Sci. 2023. PMID: 37083041
-
Integrin-mediated cell adhesion: the cytoskeletal connection.Biochem Soc Symp. 1999;65:79-99. Biochem Soc Symp. 1999. PMID: 10320934 Review.
-
Integrins Modulate T Cell Receptor Signaling by Constraining Actin Flow at the Immunological Synapse.Front Immunol. 2018 Jan 18;9:25. doi: 10.3389/fimmu.2018.00025. eCollection 2018. Front Immunol. 2018. PMID: 29403502 Free PMC article.
Cited by
-
Manganese superoxide dismutase promotes interaction of actin, S100A4 and Talin, and enhances rat gastric tumor cell invasion.J Clin Biochem Nutr. 2015 Jul;57(1):13-20. doi: 10.3164/jcbn.14-146. Epub 2015 May 22. J Clin Biochem Nutr. 2015. PMID: 26236095 Free PMC article.
-
Minimal synthetic cells to study integrin-mediated adhesion.Angew Chem Int Ed Engl. 2015 Oct 12;54(42):12472-8. doi: 10.1002/anie.201503184. Epub 2015 Aug 7. Angew Chem Int Ed Engl. 2015. PMID: 26257266 Free PMC article.
References
-
- Albelda S M, Buck C A. FASEB J. 1990;4:2868–2880. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
