Sublattice asymmetry of impurity doping in graphene: A review
- PMID: 25161855
- PMCID: PMC4142872
- DOI: 10.3762/bjnano.5.133
Sublattice asymmetry of impurity doping in graphene: A review
Abstract
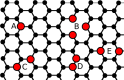
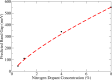
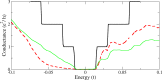
In this review we highlight recent theoretical and experimental work on sublattice asymmetric doping of impurities in graphene, with a focus on substitutional nitrogen dopants. It is well known that one current limitation of graphene in regards to its use in electronics is that in its ordinary state it exhibits no band gap. By doping one of its two sublattices preferentially it is possible to not only open such a gap, which can furthermore be tuned through control of the dopant concentration, but in theory produce quasi-ballistic transport of electrons in the undoped sublattice, both important qualities for any graphene device to be used competetively in future technology. We outline current experimental techniques for synthesis of such graphene monolayers and detail theoretical efforts to explain the mechanisms responsible for the effect, before suggesting future research directions in this nascent field.
Keywords: band gap; electronic transport; graphene; nitrogen doping; sublattice asymmetry.
Figures

Similar articles
-
Controlling Nitrogen Doping in Graphene with Atomic Precision: Synthesis and Characterization.Nanomaterials (Basel). 2019 Mar 12;9(3):425. doi: 10.3390/nano9030425. Nanomaterials (Basel). 2019. PMID: 30871112 Free PMC article. Review.
-
Large-Scale Sublattice Asymmetry in Pure and Boron-Doped Graphene.Nano Lett. 2016 Jul 13;16(7):4535-43. doi: 10.1021/acs.nanolett.6b01795. Epub 2016 Jun 6. Nano Lett. 2016. PMID: 27248659
-
Segregation of sublattice domains in nitrogen-doped graphene.J Am Chem Soc. 2014 Jan 29;136(4):1391-7. doi: 10.1021/ja408463g. Epub 2014 Jan 15. J Am Chem Soc. 2014. PMID: 24392951
-
The influence of Gaussian strain on sublattice selectivity of impurities in graphene.J Phys Condens Matter. 2016 Jun 15;28(23):235001. doi: 10.1088/0953-8984/28/23/235001. Epub 2016 May 10. J Phys Condens Matter. 2016. PMID: 27160256
-
Graphene doping methods and device applications.J Nanosci Nanotechnol. 2014 Feb;14(2):1120-33. doi: 10.1166/jnn.2014.9118. J Nanosci Nanotechnol. 2014. PMID: 24749416 Review.
Cited by
-
Controlling Nitrogen Doping in Graphene with Atomic Precision: Synthesis and Characterization.Nanomaterials (Basel). 2019 Mar 12;9(3):425. doi: 10.3390/nano9030425. Nanomaterials (Basel). 2019. PMID: 30871112 Free PMC article. Review.
-
Atomic scale interface design and characterisation.Beilstein J Nanotechnol. 2015 Aug 10;6:1708-11. doi: 10.3762/bjnano.6.174. eCollection 2015. Beilstein J Nanotechnol. 2015. PMID: 26425422 Free PMC article. No abstract available.
-
Inkjet-Printed Molybdenum Disulfide and Nitrogen-Doped Graphene Active Layer High On/Off Ratio Transistors.Molecules. 2020 Feb 28;25(5):1081. doi: 10.3390/molecules25051081. Molecules. 2020. PMID: 32121080 Free PMC article.
-
Finite-size correction scheme for supercell calculations in Dirac-point two-dimensional materials.Sci Rep. 2018 Jun 19;8(1):9348. doi: 10.1038/s41598-018-27632-6. Sci Rep. 2018. PMID: 29921873 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
