Demethionylation of Pro-1 variants of 4-oxalocrotonate tautomerase in Escherichia coli by co-expression with an engineered methionine aminopeptidase
- PMID: 25161874
- PMCID: PMC4141196
- DOI: 10.1016/j.fob.2014.07.003
Demethionylation of Pro-1 variants of 4-oxalocrotonate tautomerase in Escherichia coli by co-expression with an engineered methionine aminopeptidase
Abstract
4-Oxalocrotonate tautomerase (4-OT) catalyzes the enol-keto tautomerization of 2-hydroxymuconate, utilizing its N-terminal proline (Pro-1) as general base catalyst. Substituting Pro-1 with bulky or charged residues will result in poor or no post-translational removal of the translation-initiating methionine by the methionine aminopeptidase (MetAP) of the Escherichia coli expression host. Here, we set out to investigate whether co-expression with previously engineered aminopeptidase MetAP-∗TG can be used to produce the P1S, P1H and P1Q variants of 4-OT in a demethionylated form. The P1S variant, which carries a small residue at the penultimate position (the first position after the initiating methionine), was found to be fully processed by wild-type MetAP. The P1S variant has low-level 2-hydroxymuconate tautomerase and promiscuous oxaloacetate decarboxylase activity. The P1Q and P1H variants of 4-OT, which carry bulky residues at the penultimate position, could only be obtained in a demethionylated form (a minor fraction of the purified protein is still composed of methionylated enzyme) by co-expression with MetAP-∗TG. Interestingly, the Gln-1 residue of the demethionylated P1Q variant undergoes intramolecular cyclization to form pyroglutamate (pE), yielding variant P1pE. Whereas the P1H/M1P2H mixture has low-level tautomerase activity, the P1pE/M1P2Q mixture has robust tautomerase activity. The substitution of Pro-1 by Gln, followed by removal of the initiating Met and cyclization of Gln-1 to form pE, is a unique way to obtain a structural analogue of proline on the N-terminus of 4-OT. This opens up new possibilities to study the importance of Pro-1 in recently discovered C-C bond-forming activities of this highly promiscuous tautomerase.
Keywords: 4-Oxalocrotonate tautomerase; 5-Oxoproline; Demethionylation; Methionine aminopeptidase; Pyroglutamate.
Figures
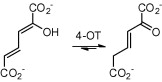
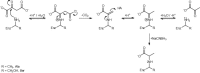
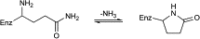
Similar articles
-
Kinetic and stereochemical analysis of YwhB, a 4-oxalocrotonate tautomerase homologue in Bacillus subtilis: mechanistic implications for the YwhB- and 4-oxalocrotonate tautomerase-catalyzed reactions.Biochemistry. 2007 Oct 23;46(42):11919-29. doi: 10.1021/bi701231a. Epub 2007 Sep 29. Biochemistry. 2007. PMID: 17902707 Free PMC article.
-
Removal of N-terminal methionine from recombinant proteins by engineered E. coli methionine aminopeptidase.Protein Sci. 2004 Jul;13(7):1802-10. doi: 10.1110/ps.04679104. Protein Sci. 2004. PMID: 15215523 Free PMC article.
-
Kinetic and structural characterization of a heterohexamer 4-oxalocrotonate tautomerase from Chloroflexus aurantiacus J-10-fl: implications for functional and structural diversity in the tautomerase superfamily.Biochemistry. 2010 Jun 22;49(24):5016-27. doi: 10.1021/bi100502z. Biochemistry. 2010. PMID: 20465238 Free PMC article.
-
The 4-oxalocrotonate tautomerase family of enzymes: how nature makes new enzymes using a beta-alpha-beta structural motif.Arch Biochem Biophys. 2002 Jun 1;402(1):1-13. doi: 10.1016/S0003-9861(02)00052-8. Arch Biochem Biophys. 2002. PMID: 12051677 Review.
-
Identification and characterization of new family members in the tautomerase superfamily: analysis and implications.Arch Biochem Biophys. 2014 Dec 15;564:189-96. doi: 10.1016/j.abb.2014.08.019. Epub 2014 Sep 16. Arch Biochem Biophys. 2014. PMID: 25219626 Free PMC article. Review.
Cited by
-
Using mutability landscapes of a promiscuous tautomerase to guide the engineering of enantioselective Michaelases.Nat Commun. 2016 Mar 8;7:10911. doi: 10.1038/ncomms10911. Nat Commun. 2016. PMID: 26952338 Free PMC article.
-
Substituting the catalytic proline of 4-oxalocrotonate tautomerase with non-canonical analogues reveals a finely tuned catalytic system.Sci Rep. 2019 Feb 25;9(1):2697. doi: 10.1038/s41598-019-39484-9. Sci Rep. 2019. PMID: 30804446 Free PMC article.
-
Engineering a Promiscuous Tautomerase into a More Efficient Aldolase for Self-Condensations of Linear Aliphatic Aldehydes.Chembiochem. 2017 Jul 18;18(14):1435-1441. doi: 10.1002/cbic.201700121. Epub 2017 May 30. Chembiochem. 2017. PMID: 28426139 Free PMC article.
References
-
- Whitman C.P., Aird B.A., Gillespie W.R., Stolowich N.J. Chemical and enzymic ketonization of 2-hydroxymuconate, a conjugated enol. J. Am. Chem. Soc. 1991;113:3154–3162.
-
- Chen L.H., Kenyon G.L., Curtin F., Harayama S., Bembenek M.E., Hajipour G., Whitman C.P. 4-Oxalocrotonate tautomerase, an enzyme composed of 62 amino acid residues per monomer. J. Biol. Chem. 1992;267:17716–17721. - PubMed
-
- Whitman C.P. The 4-oxalocrotonate tautomerase family of enzymes: how nature makes new enzymes using a beta-alpha-beta structural motif. Arch. Biochem. Biophys. 2002;402:1–13. - PubMed
-
- Poelarends G.J., Whitman C.P. Evolution of enzymatic activity in the tautomerase superfamily: mechanistic and structural studies of the 1,3-dichloropropene catabolic enzymes. Bioorg. Chem. 2004;32:376–392. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

