Transgenic rat model of Huntington's disease: a histopathological study and correlations with neurodegenerative process in the brain of HD patients
- PMID: 25162006
- PMCID: PMC4137602
- DOI: 10.1155/2014/291531
Transgenic rat model of Huntington's disease: a histopathological study and correlations with neurodegenerative process in the brain of HD patients
Abstract
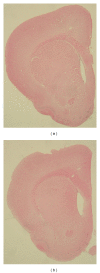
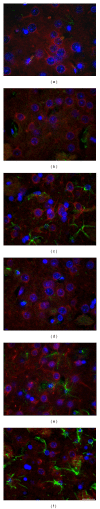
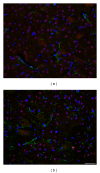
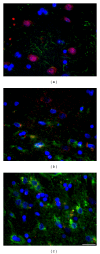
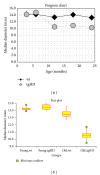
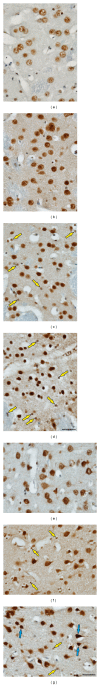
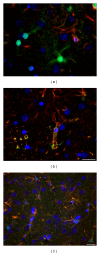
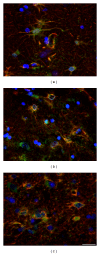
Rats transgenic for Huntington's disease (tgHD51 CAG rats), surviving up to two years, represent an animal model of HD similar to the late-onset form of human disease. This enables us to follow histopathological changes in course of neurodegenerative process (NDP) within the striatum and compare them with postmortem samples of human HD brains. A basic difference between HD pathology in human and tgHD51 rats is in the rate of NDP progression that originates primarily from slow neuronal degeneration consequently resulting in lesser extent of concomitant reactive gliosis in the brain of tgHD51 rats. Although larger amount of striatal neurons displays only gradual decrease in their size, their number is significantly reduced in the oldest tgHD51 rats. Our quantitative analysis proved that the end of the first year represents the turn in the development of morphological changes related to the progression of NDP in tgHD51 rats. Our data also support the view that all types of CNS glial cells play an important, irreplaceable role in NDP. To the best of our knowledge, our findings are the first to document that tgHD51 CAG rats can be used as a valid animal model for detailed histopathological studies related to HD in human.
Figures




Similar articles
-
The neurodegenerative process in a neurotoxic rat model and in patients with Huntington's disease: histopathological parallels and differences.Acta Histochem. 2011 Dec;113(8):783-92. doi: 10.1016/j.acthis.2010.11.007. Epub 2010 Dec 28. Acta Histochem. 2011. PMID: 21193226
-
Transgenic mice expressing mutated full-length HD cDNA: a paradigm for locomotor changes and selective neuronal loss in Huntington's disease.Philos Trans R Soc Lond B Biol Sci. 1999 Jun 29;354(1386):1035-45. doi: 10.1098/rstb.1999.0456. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10434303 Free PMC article.
-
Dendritic spine pathology and deficits in experience-dependent dendritic plasticity in R6/1 Huntington's disease transgenic mice.Eur J Neurosci. 2004 May;19(10):2799-807. doi: 10.1111/j.0953-816X.2004.03374.x. Eur J Neurosci. 2004. PMID: 15147313
-
Huntington's disease: molecular basis of neurodegeneration.Expert Rev Mol Med. 2003 Aug 22;5(20):1-21. doi: 10.1017/S1462399403006549. Expert Rev Mol Med. 2003. PMID: 14585171 Review.
-
Neurodegenerative processes in Huntington's disease.Cell Death Dis. 2011 Nov 10;2(11):e228. doi: 10.1038/cddis.2011.112. Cell Death Dis. 2011. PMID: 22071633 Free PMC article. Review.
Cited by
-
Modern approaches for modelling dystonia and Huntington's disease in vitro and in vivo.Int J Exp Pathol. 2019 Apr;100(2):64-71. doi: 10.1111/iep.12320. Epub 2019 May 15. Int J Exp Pathol. 2019. PMID: 31090117 Free PMC article. Review.
-
The Isotropic Fractionator as a Tool for Quantitative Analysis in Central Nervous System Diseases.Front Cell Neurosci. 2016 Aug 5;10:190. doi: 10.3389/fncel.2016.00190. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27547177 Free PMC article.
References
-
- Vonsattel JPG, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr. Neuropathological classification of Huntington's disease. Journal of Neuropathology and Experimental Neurology. 1985;44(6):559–577. - PubMed
-
- Gusella JF. Encyclopedia of Life Sciences. Nature Publishing Group; 2001. Huntington disease. http://www.els.net.
-
- Gusella JF, Wexler NS, Conneally PM, et al. A polymorphic DNA marker genetically linked to Huntington’s disease. Nature. 1983;306(5940):234–238. - PubMed
-
- MacDonald ME, Ambrose CM, Duyao MP, et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72(6):971–983. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

