Aedesin: structure and antimicrobial activity against multidrug resistant bacterial strains
- PMID: 25162372
- PMCID: PMC4146511
- DOI: 10.1371/journal.pone.0105441
Aedesin: structure and antimicrobial activity against multidrug resistant bacterial strains
Abstract
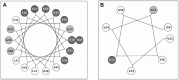
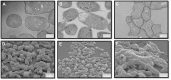
Multidrug resistance, which is acquired by both Gram-positive and Gram-negative bacteria, causes infections that are associated with significant morbidity and mortality in many clinical settings around the world. Because of the rapidly increasing incidence of pathogens that have become resistant to all or nearly all available antibiotics, there is a need for a new generation of antimicrobials with a broad therapeutic range for specific applications against infections. Aedesin is a cecropin-like anti-microbial peptide that was recently isolated from dengue virus-infected salivary glands of the Aedes aegypti mosquito. In the present study, we have refined the analysis of its structural characteristics and have determined its antimicrobial effects against a large panel of multidrug resistant bacterial strains, directly isolated from infected patients. Based the results from nuclear magnetic resonance spectroscopy analysis, Aedesin has a helix-bend-helix structure typical for a member of the family of α-helix anti-microbial peptides. Aedesin efficiently killed Gram-negative bacterial strains that display the most worrisome resistance mechanisms encountered in the clinic, including resistance to carbapenems, aminoglycosides, cephalosporins, 4th generation fluoroquinolones, folate inhibitors and monobactams. In contrast, Gram-positive strains were insensitive to the lytic effects of the peptide. The anti-bacterial activity of Aedesin was found to be salt-resistant, indicating that it is active under physiological conditions encountered in body fluids characterized by ionic salt concentrations. In conclusion, because of its strong lytic activity against multidrug resistant Gram-negative bacterial strains displaying all types of clinically relevant resistance mechanisms known today, Aedesin might be an interesting candidate for the development of alternative treatment for infections caused by these types of bacteria.
Conflict of interest statement
Figures



Similar articles
-
Synergistic activity profile of an antimicrobial peptide against multidrug-resistant and extensively drug-resistant strains of Gram-negative bacterial pathogens.J Pept Sci. 2017 Apr;23(4):329-333. doi: 10.1002/psc.2978. Epub 2017 Feb 8. J Pept Sci. 2017. PMID: 28176481
-
Beta-defensin derived cationic antimicrobial peptides with potent killing activity against gram negative and gram positive bacteria.BMC Microbiol. 2018 Jun 5;18(1):54. doi: 10.1186/s12866-018-1190-z. BMC Microbiol. 2018. PMID: 29871599 Free PMC article.
-
Effects of Aib residues insertion on the structural-functional properties of the frog skin-derived peptide esculentin-1a(1-21)NH2.Amino Acids. 2017 Jan;49(1):139-150. doi: 10.1007/s00726-016-2341-x. Epub 2016 Oct 11. Amino Acids. 2017. PMID: 27726008
-
Antimicrobial AApeptides.Curr Top Med Chem. 2017;17(11):1266-1279. doi: 10.2174/1568026616666161018145945. Curr Top Med Chem. 2017. PMID: 27758686 Free PMC article. Review.
-
An intimate link between antimicrobial peptide sequence diversity and binding to essential components of bacterial membranes.Biochim Biophys Acta. 2016 May;1858(5):958-70. doi: 10.1016/j.bbamem.2015.10.011. Epub 2015 Oct 21. Biochim Biophys Acta. 2016. PMID: 26498397 Review.
Cited by
-
Unraveling dual feeding associated molecular complexity of salivary glands in the mosquito Anopheles culicifacies.Biol Open. 2015 Jul 10;4(8):1002-15. doi: 10.1242/bio.012294. Biol Open. 2015. PMID: 26163527 Free PMC article.
-
Anti-inflammatory activities of Aedes aegypti cecropins and their protection against murine endotoxin shock.Parasit Vectors. 2018 Aug 14;11(1):470. doi: 10.1186/s13071-018-3000-8. Parasit Vectors. 2018. PMID: 30107813 Free PMC article.
-
In Silico Structural Evaluation of Short Cationic Antimicrobial Peptides.Pharmaceutics. 2018 Jun 21;10(3):72. doi: 10.3390/pharmaceutics10030072. Pharmaceutics. 2018. PMID: 29933540 Free PMC article.
-
Antimicrobial Effect of a Peptide Containing Novel Oral Spray on Streptococcus mutans.Biomed Res Int. 2020 Mar 10;2020:6853652. doi: 10.1155/2020/6853652. eCollection 2020. Biomed Res Int. 2020. PMID: 32258136 Free PMC article.
-
Application of Antibiotics/Antimicrobial Agents on Dental Caries.Biomed Res Int. 2020 Jan 31;2020:5658212. doi: 10.1155/2020/5658212. eCollection 2020. Biomed Res Int. 2020. PMID: 32076608 Free PMC article. Review.
References
-
- Diene SM, Rolain JM (2013) Investigation of antibiotic resistance in the genomic era of multidrug-resistant Gram-negative bacilli, especially Enterobacteriaceae, Pseudomonas and Acinetobacter. Expert Rev Anti Infect Ther 11: 277–296. - PubMed
-
- Nigam A, Gupta D, Sharma A (2014) Treatment of infectious disease: Beyond antibiotics. Microbiol Res. - PubMed
-
- Hoffmann JA (2003) The immune response of Drosophila. Nature 426: 33–38. - PubMed
-
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA (2007) The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol 7: 862–874. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

