A synaptic and circuit basis for corollary discharge in the auditory cortex
- PMID: 25162524
- PMCID: PMC4248668
- DOI: 10.1038/nature13724
A synaptic and circuit basis for corollary discharge in the auditory cortex
Abstract
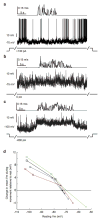
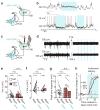
Sensory regions of the brain integrate environmental cues with copies of motor-related signals important for imminent and ongoing movements. In mammals, signals propagating from the motor cortex to the auditory cortex are thought to have a critical role in normal hearing and behaviour, yet the synaptic and circuit mechanisms by which these motor-related signals influence auditory cortical activity remain poorly understood. Using in vivo intracellular recordings in behaving mice, we find that excitatory neurons in the auditory cortex are suppressed before and during movement, owing in part to increased activity of local parvalbumin-positive interneurons. Electrophysiology and optogenetic gain- and loss-of-function experiments reveal that motor-related changes in auditory cortical dynamics are driven by a subset of neurons in the secondary motor cortex that innervate the auditory cortex and are active during movement. These findings provide a synaptic and circuit basis for the motor-related corollary discharge hypothesized to facilitate hearing and auditory-guided behaviours.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




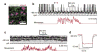






Comment in
-
Sensory systems: Sound processing takes motor control.Nature. 2014 Sep 11;513(7517):180-1. doi: 10.1038/nature13658. Epub 2014 Aug 27. Nature. 2014. PMID: 25162533 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

